Scroll to:
Development and evaluation of a reagent set for quantitation of mRNA expression level of chimeric BCR::ABL1 gene
https://doi.org/10.47183/mes.2024-244
Abstract
Introduction. Chronic myeloid leukemia is a myeloproliferative disease associated with a t(9;22)(q34;q11) translocation resulting in a chimeric BCR::ABL1 gene. Molecular genetic studies are used as a diagnostic method and for determination of minimal residual disease by quantification of the mRNA expression level of the chimeric BCR::ABL1 gene.
Objective. To validate the developed multiplex test system for simultaneous quantitative determination of e13a2 (b2a2) and e14a2 (b3a2) transcripts of translocation p210 in comparison with the analog system registered in Russia.
Materials and methods. In total, 50 peripheral blood samples were used. Of these, 39 belonged to patients diagnosed with CML; 11 blood samples from healthy people were used to confirm analytical specificity. The reagent kit includes the specifically developed primers and fluorescently labeled probes, the concentration of which was selected experimentally, as well as original reagents manufactured by the Centre for Strategic Planning, Federal Medical and Biological Agency (Russia). Statistical analysis was performed using the StatTech v.4.5.0 software (developed by StatTech, Russia). Differences were considered significant at p < 0.05.
Results. The conducted analytical and diagnostic characterization of the developed kit showed the following results. The relative sensitivity determined using cell standards was 0.01 %. The reproducible analytical sensitivity was 100 copies/mL (CV = 1.86 %). In analytical specificity testing, the absence of false results was shown. When testing the reagents of the test system on clinical samples, the complete convergence (100 %) with the results obtained using the reference kit was revealed.
Conclusions. Approbation of the developed reagent set showed its sufficiently high analytical sensitivity and diagnostic sensitivity. This set can be used for identification and quantitative determination of the mRNA of the chimeric BCR::ABL1 gene for the purposes of diagnosis, timely and accurate therapy, and monitoring of minimal residual disease.
For citations:
Avdonina M.A., Kuklina N.G., Glavatskaya A.A., Dmitriev V.K., Chegodar A.S., Danishevich A.M., Bodunova N.A., Abramov I.S., Shipulin G.A. Development and evaluation of a reagent set for quantitation of mRNA expression level of chimeric BCR::ABL1 gene. Extreme Medicine. 2025;27(1):74-79. https://doi.org/10.47183/mes.2024-244
INTRODUCTION
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm caused by malignant degeneration of hematopoietic stem cells. This disease is characterized by increased proliferation of granulocytic germ cells without the loss of their differentiation ability, hyperplasia of myeloid tissue, myeloid metaplasia of hematopoietic organs. These disorders are associated with a chromosomal abnormality, i.e., t(9;22)(q34;q11) translocation or the so-called Philadelphia chromosome, as a result of which the chimeric BCR::ABL1 gene is formed [1]. This fusion is the most common cytogenetic abnormality in adult patients with chronic myeloid leukemia (CML) in 95% of cases, as well as with acute lymphoblastic leukemia (ALL) in 30% of adults and about 5% of children1 [1].
According to the statistical data for 2022, 1087 new cases of CML were detected in Russia, with about 54% women and 47% men. This nosology is most frequently (in 28.9% of cases) registered in people aged 60–69 years. In 16.5% of cases, CML was detected in people aged 50–59 years and 70–79 years. The incidence of CML in people aged 30–39 years equals about 12% [2].
The mentioned translocation is diagnosed by cytogenetic examination (karyotype) of the bone marrow or molecular cytogenetic examination (FISH method), as well as by determining the quantitative level of expression of BCR::ABL1 p210 mRNA for e13a2 or e14a2 transcripts.2
Modern therapy is aimed at achieving a major molecular response (MMR>3.0), a deep molecular response (DMR≥4.0), as well as at the subsequent prevention of the appearance of tumor clones [3][4]. Several assessment methods can be used to monitor the response to treatment with tyrosine kinase inhibitors (TKI), such as a complete hematologic response, determined by examining the complete blood count; a partial hematologic response, determined by flow cytometry; and a complete cytological response, evaluated using bone marrow aspirate and biopsy samples based on studying the molecular response using quantitative PCR [5].
PCR is known to be the most sensitive method for assessing minimal residual disease; however, the diversity of reagent kits from different manufacturers results in a high interlaboratory variability in the results obtained. This also leads to inconsistencies in the compared indicators, thus affecting the selection of appropriate treatment tactics. According to the recommendations of the European LeukemiaNet and the National Comprehensive Cancer Network, CML molecular monitoring can be harmonized by monitoring the BCR::ABL1 mRNA levels using the international scale (IS), which is based on the standardized basic transcript level presented in the International Randomized Study of Interferon and STI571–IRIS [3]. According to the results obtained, this study proposed to evaluate the logarithmic decrease in BCR::ABL1 (IS ratio) during therapy in comparison with the initial IRIS ratio at diagnosis [6][7]. To be able to apply this International Scale, each laboratory must calibrate the results obtained using a conversion factor (CF), the numerical value of which must be multiplied by the quantitative indicators obtained in a particular laboratory. In order to determine the CF, which is individual for each laboratory, the World Health Organization has created an international genetic panel for quantification of BCR::ABL1 transcripts using PCR, containing four different variants (10%, 1%, 0.1%, 0.01%), using the K562 cell line diluted in the cell line, the BCR::ABL1 merger code [9].
According to the recommendations of International Guidelines for the diagnosis of BCR::ABL1 mRNA expression and monitoring of minimal residual disease, it is necessary to use the quantitative reverse transcription PCR (RT-PCR) method with a detection sensitivity lower than MO 4.5 (0.0032% IS) [4][9]. The use of reagents with insufficient sensitivity can lead to incorrect test results and, as a result, premature termination of treatment and further progression of the disease [6]. According to Clinical Recommendations, the procedure for periodic monitoring of expression levels has been define as follows. When the expression level of p210 BCR::ABL1 mRNA is below the BMO, quantitative real–time PCR is performed every three months. After reaching the BMO, the assay is performed every six months [10][11]. In this regard, the development, testing, and implementation into clinical and laboratory practice of domestic highly sensitive specific diagnostic reagent kits for determining the level of BCR::ABL1 transcripts using PCR present a relevant research task.
In this work, we set out to develop and evaluate a set of reagents for quantitative determination of the mRNA expression level of the chimeric BCR::ABL1 gene in peripheral blood samples.
MATERIALS AND METHODS
The study included 50 people aged 20 to 80 years, including 28 men and 22 women. The main group consisted of 39 patients with the established clinical diagnosis of CML. In order to validate the analytical specificity for the absence of false positive results, blood samples from 11 apparently healthy individuals, comparable in gender and age with the main group of patients, were used.
Peripheral blood samples were obtained at the Loginov Moscow Clinical Scientific Centre; all the participants were requested to sign an informed consent. Blood sampling was carried out in vacuum tubes with K2 EDTA (China). To confirm the diagnosis of CML and determine the mRNA expression level of the chimeric gene BCR::ABL1, a set of reagents was used to detect and quantify the BCR::ABL1 chimeric gene mRNA (M-bcr variant) and the abl gene mRNA in clinical material by real-time PCR with fluorescence detection AmpliSens® Leukemia Quantum M-bcr-FRT (Central Research Institute of Epidemiology, Russia) according to the manufacturer’s instructions.
RNA was isolated using a set of AmpliTest® RIBO-prep reagents (Centre for Strategic Planning, FMBA, Russia) according to the instructions. The efficiency of mRNA isolation was determined using a Qubit 4 fluorimeter (Thermo Scientific, USA).
The developed reagent kit includes specific primers and fluorescently labeled probes, the presence of which allows simultaneous detection of the expression of the chimeric BCR::ABL1 gene and the ABL1 gene. The oligonucleotides for detecting chimeric gene expression were selected based on the recommendations of the international group Europe Against Cancer (EAC) in 2003 and allowed the identification of the two most common chimeric transcripts, i.e., e13a2 (b2a2) and e14a2 (b3a2). The control of material collection, RNA isolation, reverse transcription reaction, and PCR was carried out using endogenous internal control, which used specifically selected primers and a probe for the ABL1 gene.
The concentrations of primers and fluorescently labeled probes were selected experimentally.
The reaction mixture was prepared using the following reagents (manufactured by Centre for Strategic Planning, FMBA, Russia): 5x PCR buffer (5 µL), 10 mM dNTP (0.5 µL), Taq polymerase (0.5 µL), and MMLV revertase (0.25 µL).
PCR amplification conditions were as follows: reverse transcription at 50°C (30 min), preheating at 95°C (15 min), followed by 45 cycles of denaturation at 95°C (10 sec) and annealing at 60°C (60 sec). The fluorescent signal was detected via the FAM and HEX channel.
Interpretation was carried out only for correct results for a negative control sample (NCS) and a positive control sample (PCS) at each setting. Sterile deionized water was used as a NCS; PCS was a mixture of bacteriophage preparations containing sequences of mRNA translocation p210 of the chimeric gene BCR::ABL1 and mRNA of the control gene ABL1.
Plasmids containing mRNA sequences of translocation p210 of the chimeric BCR::ABL1 gene and mRNA of the control ABL1 gene with a known concentration were used as calibrators. The concentration of plasmids was measured using the QX200 digital PCR system (Bio-Rad, USA). Plasmids were used in five 10-fold dilutions from about 10 to 1×106 copies/mL to determine the linearity and effectiveness of RT-PCR. A single-stage RT-PCR reaction was performed on a DT-96 amplifier (DNA Technology, Russia).
To establish the detection limit for the specificity and sensitivity of the developed kit, RNA isolated from the K562 cell line (BCR::ABL1 — positive) and HeLa (wild-type samples), as well as samples from CML patients and healthy donors, were used. To determine the relative sensitivity, cellular standards with certain concentrations were prepared, namely 10%, 1%, 0.1%, and 0.01% according to the procedure described by White et al., 2010 [8]. The number of cells was calculated using an automatic Countess II FL Automated Cell Counter (Thermo FS). The resulting standards were calibrated according to the WHO protocol [12].
The relative mRNA expression level of the chimeric BCR::ABL1 gene was estimated based on calculations for BCR::ABL1/ABL1 using the following formula:
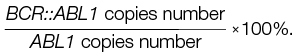
Statistical analysis was performed using the StatTech v.4.5.0 software (developed by Stattech LLC, Russia). Differences were considered significant at p < 0.05.
RESULTS AND DISCUSSION
Testing of the reagent kit developed in this study produced the following results. Real-time PCR parameters were as follows: efficiency ranging within 95–105% and the correlation coefficient r = 0.99. According to experimental data using cellular standards, the relative sensitivity of the developed set was 0.01%, which corresponds to the level of complete molecular response (CMR)3. The reproducible sensitivity for control plasmids and for RNA isolated from K562 cell culture was 100 copies/mL (CV% = 1.86).
Verification of the analytical specificity showed the absence of false positive results when testing samples obtained from healthy patients. During testing, only the internal control signal via the HEX channel was detected; the mRNA detection signal for the chimeric BCR::ABL1 gene in the FAM channel was absent.
In this study, we analyzed a random sample of peripheral blood samples from patients with the clinical diagnosis of CML. In the main group, mRNA expression of translocation p210 of the chimeric BCR::ABL1 gene was detected in all 39 samples. The number of positive results was 21 (53.8%) and 18 (46.2%) among men and women, respectively. The incidence of translocation in the age groups of 20–29, 30–49, 50–59, 60–69 and 70–79, and over 80 years сomprised 3.2, 12.9, 9.6, 19.3, and 6.4% of cases, respectively, which agrees well with statistical data. The largest number of patients with these changes were registered in the age group of 60–69 and 70–79 years, which is also consistent with statistical data for Russia [2]. Patients under the age of 20 years were not represented due to their limited sample. Morbidity at this age equals about 1% nationwide. CML is equally common in both men and women [13][14].
The relative expression of the BCR::ABL1 gene (protein p210, variants b3a2 or b2a2) according to the results of testing in peripheral blood samples of patients with the clinical diagnosis of CML ranged from 2.3% to 100%. The number of copies of the ABL1 gene ranged from 2200 copies/mL to 17,595,440 copies/mL in the CML group and from 11,675,896 copies/mL to 18,634,577 copies/mL in the control group.
According to the Laboratory Guidelines for the Diagnosis and Treatment of Chronic Myeloid Leukemia (European LeukemiaNet), the minimum number of reference gene transcripts, regardless of whether BCR::ABL1 is detected or not, should be at least 10,000 ABL1 for MR 4, 32,000 for MR 4.5, and 100,000 for MR 5 in the same volume of cDNA in which the sample is being tested for BCR::ABL1 [4]. The number of transcripts of the reference gene obtained meets international standards; therefore, the results can be included in clinical reports.
During testing of CML patients, eight samples of IS<1% BCR were identified: ABL1 (MR 2), nine samples of IS<0.1% (MR 3), seven samples of IS<0.01% (MR 4), seven samples of IS<0.0032% (MR 4.5). The object of the study was blood samples from randomized patients. The criterion for inclusion in the main group of patients was the presence of the confirmed diagnosis of CML, established using the AmpliSens® Leukemia Quantum M-bcr-FRT analogue registered in Russia.
Testing of the developed reagent kit on peripheral blood samples with a known level of expression of the chimeric BCR::ABL1 gene demonstrated their complete agreement with the results obtained using the AmpliSens® Leukemia Quantum M-bcr-FRT reagent kit.
When establishing the diagnosis of CML, molecular genetic analysis (FISH method) is used to identify the chimeric BCR::ABL1 gene, as well as to determine the expression of BCR::ABL1 p210 mRNA (quantitative) for chimeric e13a2 or e14a2 transcripts by PCR [15]. This method is also used in assessing the level of response to treatment of patients with tyrosine kinase inhibitors (TKI) and determining the level of minimal residual disease when the number of residual leukemic cells is below the sensitivity level of cytogenetic studies [16].
The current market offers a number of domestic test systems designed to detect the mRNA of the chimeric BCR::ABL1 gene. These include, e.g., AmpliSens® Leukemia Quantum M-bcr-FRT (Central Research Institute of Epidemiology of Rospotrebnadzor), ONCOSCREEN 1-1-Q (GenoTechnology), with and without registration certificates: Myeloscreen BCR-ABL (Gene Formula), BCR-ABL1 Mbcr RQ Kit® (“Inogen”).
In terms of the number of reproducible copies, the analytical sensitivity of the AmpliSens® Leukemia Quantum M-bcr-FRT kit was twice as low as that of the analyzed kit, amounting to 237 copies/mL vs 100 copies/mL.4 At the same time, the analysis using the AmpliSens® Leukemia Quantum M-bar-FRT kit involves setting up a reverse transcription reaction initially in isolation, and then performing real-time PCR, which is quite laborious. Unlike the reference kit, the developed kit used a mixture that allows detecting the expression of BCR::ABL1 p210 mRNA in one tube.
The results of quantification of BCR::ABL1 mRNA obtained using the kit under study were similar to those recorded using the AmpliSens® Leukemia Quantum M-bcr-FRT kit in all samples. Both sets are capable of identifying at least a 4.5-fold decrease in the IS ratio.
The BCR/ABL MULTITEST kit can be used to simultaneously detect chimeric transcripts p210, p190, and p230 of the BCR::ABL1 gene using the RT-PCR multiplex format, which is economically feasible during primary screening [17].
In 2019, Kitamura et al. presented a new high-sensitive two-stage reagent kit RT-PCR (Reverse Transcription Polymerase Chain Reaction) with a diagnostic sensitivity of 0.01%, which was determined using the Armored RNA Quant® (ARQ) secondary control panel (Asuragen, Inc., Austin, Texas, USA) [6]. Dubina et al. determined the analytical parameters of the test, in which the specificity should strive for 100%, adequate for the sensitivity level of 3–5 copies of mRNA to the reaction, while the ratio of the BCR::ABL1 transcript to the normalizer gene should be 0.01% (corresponding to the level of CMR) [18].
In comparison with its analog system, the developed kit has a number of advantages. Firstly, it exhibits higher analytical indicators. Secondly, the use of single-stage RT-PCR significantly reduces the risk of contamination and technical errors during the excavation of reagents and samples, while also reducing the working time of personnel. Finally, the multiplex mixture of specific probes and primers used over two detection channels can significantly reduce the amount of expendable materials used.
Thus, we have succeeded in creating a highly sensitive multiplex test system for simultaneous detection of e13a2 (b2a2) and e14a2 (b3a2) transcripts of the p210 translocation, which also assumes endogenous internal control to assess the correctness of all stages of PCR, including RNA isolation.
CONCLUSION
Quantification and detection of translocation in CML is essential for selecting adequate treatment protocols and monitoring disease recurrence. Sensitive and accurate monitoring of minimal residual disease plays an important role in managing CML, facilitating the decision-making process concerning treatment cessation and identification of patients at risk of progression. The conducted testing of the developed test system, which makes it possible to detect the p210 transcript of the chimeric BCR::ABL1 gene, showed a sufficiently high order of diagnostic specificity and sensitivity of the developed set of reagents for quantitative detection of the level of relative mRNA expression of the chimeric BCR::ABL1 gene in the blood in order to verify the diagnosis of CML at the level of 100%. The use of endogenous internal control (the ABL1 gene) made it possible to monitor the main stages of PCR (sample preparation, RNA isolation, reverse transcription, and amplification reactions).
1. Clinical Guidelines: Management of Chronic Myeloid Leukemia (CML) in adults. С92.1.
2. Ibid.
3. Clinical Guidelines: Management of Chronic Myeloid Leukemia (CML) in adults. С92.1.
4. https://roszdravnadzor.gov.ru/services/misearch
References
1. Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1043–63. https://doi.org/10.1016/j.hoc.2009.07.007
2. Kaprin AD, red. Malignant neoplasms in Russia in 2022 (morbidity and mortality). M.: P.A. Herzen Moscow State Medical Research Institute — branch of the Federal State Budgetary Institution NMIC Radiology of the Ministry of Health of Russia; 2023(In Russ.).
3. Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Aude C, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2017;35(3):298–305. https://doi.org/10.1200/JCO.2016.68.2914
4. Hochhaus A., Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F., et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia. 2020;34(4):966–84. https://doi.org/10.1038/s41375-020-0776-2
5. Kantarjian H., Schiffer С., Jones D., Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111(4): 1774–80. https://doi.org/10.1182/blood-2007-09-110189
6. Kitamura H, Tabe Y, Ai T, Tsuchiya K, Yuri M, Misawa S, et al. A new highly sensitive real-time quantitative-PCR method for detection of BCR-ABL1 to monitor minimal residual disease in chronic myeloid leukemia after discontinuation of imatinib. PLoS One. 2019;14(3):e0207170. https://doi.org/10.1371/journal.pone.0207170
7. Martinez RJ, Kang Q, Nennig D, Bailey N, Brown N, Betz B, et al. One-Step Multiplexed Droplet Digital Polymerase Chain Reaction for Quantification of p190 BCR-ABL1 Fusion Transcript in B-Lymphoblastic Leukemia. Arch Pathol Lab Med. 2022;146(1):92–100. https://doi:10.3390/diagnostics12061305
8. White H.E, Matejtschuk P, Rigsby P, Gabert J, Lin F, Wang YL, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood. 2010;116(22):e111–7. https://doi.org/10.1182/blood-2010-06-291641
9. Özdemir NZ, Kılıçaslan NA, Yılmaz M, Eşkazan AE. Guidelines for the Treatment of Chronic Myeloid Leukemia From the NCCN and ELN: Differences and Similarities. Int J Hematol. 2023;117(1):3–15. https://doi.org/10.1007/s12185-022-03446-1
10. Kottwitz D, Hadi EH, Amrani E, Cabezas S, Dehbi H, Nadifi S, et al. Evaluation of a novel multiplex RT-qPCR assay for the quantification of leukemia-associated BCR-ABL1 translocation. Int J Hematol. 2015;102(3):335–41. https://doi.org/10.1007/s12185-015-1839-4
11. Franke GN, Maier J, Wildenberger K, Cross M, Giles FJ, Müller M.C, et al. Comparison of Real-Time Quantitative PCR and Digital Droplet PCR for BCR-ABL1 Monitoring in Patients with Chronic Myeloid Leukemia Comparative Study. J Mol Diagn. 2020;22(1):81–9. https://doi.org/10.1016/j.jmoldx.2019.08.007
12. International Standard, 1st WHO International Genetic Reference Panel for the quantitation of BCR-ABL1 translocation NIBSC code: 09/138
13. Winn AN, Atallah E, Cortes J, Deininger MWN, Kota V, Larson RA, et al. Estimated Savings After Stopping Tyrosine Kinase Inhibitor Treatment Among Patients With Chronic Myeloid Leukemia. JAMA Netw Open. 2023;6(12):e2347950. https://doi.org/10.1001/jamanetworkopen.2023.47950
14. Cantoni N, Sommavilla R, Seitz P, Kulenkampff E, Kahn S, Lambert JF, et al. A multicenter real-world evidence study in the Swiss treatment landscape of chronic myeloid leukemia. BMC Cancer. 2022;22(1):1192. https://doi.org/10.1186/s12885-022-10241-y
15. Cross NCP, Ernst T, Branford S, Cayuela J-M ,Deininger M, Fabarius A,et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023;37(11):2150–67. https://doi.org/10.1038/s41375-023-02048-y
16. Ryabchikova NR, Minniakhmetov IR, Safuanova GSh, Islamgulov DV, Karunas AS, Khusnutdinova EE. Chronic myelogenous leukemia: molecular monitoring in clinical practice. Oncohematology. 2013;8(1):7–16 (In Russ.). https://doi.org/10.17650/1818-8346-2013-8-1-7-16
17. Gorbenko AS, Stolyar MA, Vasiliev EV, Mikhalev MA, Bakhtina VI, Olkhovik TI, et al. Use of the «BCR/ABL — multitest» kit in the algorithm of laboratory diagnostics of oncohematological diseases: economic aspects. Klinicheskaia Laboratornaia Diagnostika.2021;66(9):571–6. https://doi.org/10.51620/0869-2084-2021-66-9-571-576
18. Dubina MV, Kuevda DA, Khomyakova TE, Tsaur GA, Kutsev SI, Zaritskiy AY. Molecular monitoring of the effectiveness of therapy in patients with chronic myeloid leukemia in Russia. Journal of Modern Oncology. 2010; 4(12):9–17 (In Russ.). EDN: NCXVBJ
About the Authors
M. A. AvdoninaRussian Federation
Mariia A. Avdonina
Moscow
N. G. Kuklina
Russian Federation
Natalia G. Kuklina
Moscow
A. A. Glavatskaya
Russian Federation
Arina A. Glavatskaya
Moscow
V. K. Dmitriev
Russian Federation
Vladislav K. Dmitriev
Moscow
A. S. Chegodar
Russian Federation
Anzhelika S. Chegodar
Moscow
A. M. Danishevich
Russian Federation
Anastasia M. Danishevich
Moscow
N. A. Bodunova
Russian Federation
Natalia A. Bodunova
Moscow
I. S. Abramov
Russian Federation
Ivan S. Abramov
Moscow
G. A. Shipulin
Russian Federation
German A. Shipulin
Moscow
Supplementary files
Review
For citations:
Avdonina M.A., Kuklina N.G., Glavatskaya A.A., Dmitriev V.K., Chegodar A.S., Danishevich A.M., Bodunova N.A., Abramov I.S., Shipulin G.A. Development and evaluation of a reagent set for quantitation of mRNA expression level of chimeric BCR::ABL1 gene. Extreme Medicine. 2025;27(1):74-79. https://doi.org/10.47183/mes.2024-244
















