Scroll to:
Heat transfer by evaporation determines the effect of nasal phenazepam on thermal stress in rats
https://doi.org/10.47183/mes.2024-245
Abstract
Introduction. Thermal stress is an increase in body temperature due to the predominance of heat received from outside or released during metabolism over heat losses by the body. Heat production can be regulated using benzodiazepines in doses unattainable with a single intramuscular injection of their officinal preparations. In this study, this limitation is overcome using a prototype of the Phenazepam nasal spray (PNS) preparation, containing 170 mg of phenazepam in 1 mL of a non-aqueous solution.
Objective. Experimental assessment of the PNS effect on the metabolic rate and thermal balance in thermal stress.
Materials and methods. The effect of a single 10 μL PNS intranasal instillation on the external respiration intensity, oxygen consumption, as well as 10 μL PNS intranasal instillations at 0.5 h intervals on the dynamics of rectal temperature, body weight, and lethality in rats at an air temperature of 40 °C was studied.
Results. PNS instillations reduced oxygen consumption by an amount sufficient to decrease body temperature by 0.3 °C in 0.5 h. PNS administration declined the rate of body temperature rise when placing rats in restrainers at an air temperature of 40 °C; however, PNS administration accelerated body temperature rise and increased lethality when placing rats in cages. Due to PNS, moisture loss by rats in cages decreased, judged by the dynamics of body weight.
Conclusions. The study confirmed the prospects of PNS as a pharmacotherapy for heat stroke at a high relative humidity, exposure to insulating skin protectors, or with immersion hyperthermia. The possibility of the aggravating effect of PNS on human thermal stress in the absence of physical obstacles to heat transfer by evaporation requires additional verification.
Keywords
For citations:
Ivnitsky J.J., Vakunenkova O.A., Krasnov K.A., Gaft S.S., Lapina N.V. Heat transfer by evaporation determines the effect of nasal phenazepam on thermal stress in rats. . 2025;27(1):37-42. https://doi.org/10.47183/mes.2024-245
INTRODUCTION
Thermal (heat) stress is an increase in the core body temperature, when the algebraic sum of thermal energy received from outside and released in metabolic processes exceeds that of thermal energy lost by evaporation, radiation, convection, and heat conduction. The totality of the most severe clinical manifestations of heat stress, referred to as heat stroke [1], is a critical condition with a mortality rate of 27% [2]. Not only children and elderly people are at increased risk of heat stroke, but also the economically active population, whose activities are associated with physical exertion or work in conditions that impede heat transfer. These include, e.g., military personnel, police officers, firefighters, hot shop workers, and athletes.
The basic principle of first aid in heat stroke management consists in a rapid reduction of body temperature. The current standard of emergency medical care1 recommend physiotherapeutic means for promoting heat transfer, such as cooling liquids and applications. Medications include saline solutions, nonsteroidal anti-inflammatory drugs (NSAID), and diazepam. Based upon the daily or course dose of 10 mg, diazepam can be prescribed in a single dose of 5 mg as a sedative. The current literature lacks data on the effect of diazepam used in this mode on the thermal state of the body. However, parenteral administration of benzodiazepines in doses of more than 20 mg, similar to those used to relieve seizures of chemical etiology [3], is likely to produce the required effect.
Intramuscular administration of benzodiazepines at the prehospital stage, commonly applied for the purpose of relieving convulsive syndrome, is a forced alternative to their intravenous administration, which is problematic in the setting of seizures. Due to the low water solubility of pharmaceutical substances of the benzodiazepine group, their concentration in official injectable medicines (sibazone, midazolam) does not exceed 5 mg/mL. Therefore, simultaneous delivery of benzodiazepines into the human body in doses of more than 20 mg requires intramuscular administration of such medicines in volumes of more than 4 mL, which is rarely possible. We previously proposed an approach to overcoming this limitation based on the use of a non-aqueous solution of benzodiazepine [4] and its nasal dosage form [5]. A prototype of the Phenazepam Nasal Spray medicine (PNS) was created, containing 170 mg of phenazepam in 1 mL. With a double insufflation of 140 µL of PNS into each nasal passage, the dose of phenazepam exceeded the highest single doses of diazepam or midazolam administered as official injectable medicines by 4.8 and 6.4 times, respectively. The need for such doses was determined by the initial requirement for PNS as a means of relieving convulsive syndrome of chemical etiology. However, an increase in drug dose offers the possibility of phenazepam exhibiting other pharmacological properties, potentially beneficial in conditions of heat stress. In this regard, the fact that benzodiazepines reduce oxygen consumption by both the brain [6] and the body as a whole [7] deserves attention. The associated decrease in heat production could delay the increase in body temperature in conditions conducive to overheating of the body, thereby increasing tolerance against heat stress. However, the opposite effect, i.e., violation of behavioral patterns aimed at stimulating heat transfer, must not be neglected. In rats, whose skin, excluding the plantar surface of the extremities, is devoid of sweat glands, hypothetically grooming with saliva applied to the coat and its subsequent evaporation is a thermoregulatory reaction similar to sweating in humans [8].
This study aims to experimentally evaluate the effect of PNS on the metabolic rate and heat balance during heat stress.
MATERIALS AND METHODS
The study was conducted using outbred albino male rats (191–210 g) purchased from the Rappolovo Branch of the Kurchatov Institute Research Center. The animals were receiving standard rat food and drinking water ad libitum. Two series of experiments were conducted.
In the first series of experiments, 16 animals were used, eight individuals in each group (control, experiment). Changes in the intensity of respiratory function and oxygen consumption by the body were studied after a single intranasal instillation of isotonic 0.9% and sodium chloride solution in control animals and PNS 170 mg/mL in a volume of 10 µL (5 µL in each nasal passage) in animals from the experimental group, which corresponds to an average dose of the phenazepam® pharmaceutical substance 8.5 mg/kg and bioequivalent to a dose of 100 mg (four insufflations of 140 µL of PNS) for humans. Variable volume dispensers were used to administer the solutions. The acute toxicity of PNS at this dose was characterized based on the data from a preclinical study of its prototype, in which intranasal administration of PNS to rats at a 20-fold higher dose (five times 40 µL with an interval of 10 min) did not cause animal death. Body oxygen consumption was determined in a Miropolsky apparatus with a 2L respirometric chamber, to which the animals were accustomed for 2 min before the onset of each measurement. The intensity of body oxygen consumption QО2, mL/(kg×min), was found from the equation:
QО2 = V×F/(m×∆t), (1)
wherein V — the volume of manometric liquid entering the burette, mL; F — the coefficient for reducing the oxygen volume to normal conditions; m — body weight, kg; ∆t — time spent by a rat in a sealed chamber, min.
The measurement duration was 3 min under its absolute error of 0.1 mL (≤ 2% of the V value). The animals were not fixed, they were freely located in the respirometric chamber. At this time, their respiratory rate was calculated (min-1). The measurements were carried out with an interval of 10 min.
The second series of experiments studied the effect of PSN on the dynamics of rectal temperature, body weight, and mortality of rats housed in a thermal chamber in conditions that allowed (placement in enclosures) or excluded (placement in restrainers) grooming. Four randomized groups were formed: two control groups and two experimental groups with 12–14 individuals in each:
Group 1 (n = 14) — intranasal administration of 0.9% sodium chloride solution in a volume of 10 µL; animal husbandry;
Group 2 (n = 14) — intranasal injection of PSN 170 mg/mL in a volume of 10 µL; keeping animals in an aviary;
Group 3 (n = 12) — intranasal administration of 0.9% sodium chloride solution in a volume of 10 µL; animal husbandry in restrainers;
Group 4 (n = 12) — intranasal administration of 0.9% PSN solution 170 mg/mL in a volume of 10 µL; animal husbandry in restrainers.
The medications were instilled 5 µL into each nasal passage before the animals were placed in a thermal chamber, followed by instillation every half hour. The number of instillations depended on the life span of the animals and ranged from two to seven; the total dose of phenazepam ranged from 17 mg/kg to 59.5 mg/kg.
Immediately after the administration of the medications, the animals (three individuals from each group at a time) were placed in a thermal chamber. The size of the restrainers did not interfere with breathing movements, but excluded grooming. The climatic conditions that contributed to overheating of the body were modeled in a BMT Stericell SC 111 ECO thermal chamber (Czech Republic) with a volume of 111 L and exhaust ventilation of 5 m3/h. The air temperature was 40 ± 1°C, the relative humidity of 46% was maintained automatically. The thermal balance of the body was assessed by the dynamics of rectal temperature, which was measured at 30 min intervals with an electric thermometer equipped with a sensor for rats RET-2 (WPI, China); the tip of the thermometer was inserted into the rectum to a depth of 3 cm. The body loss of moisture was assessed by changes in body weight measured at half-hour intervals.
The results were presented as an average value and its error (M ± m). The Shapiro–Wilk criterion was used to check the normality of the distribution. A multidimensional analysis of variance was performed to assess the effect of thermal conditions and injected substances on the recorded quantitative indicators. In cases where any factor showed a significant influence, a one-dimensional analysis of variance was performed. In cases of experimental plans with repeated measurements, models with mixed effects were used. The intergroup comparison of averages was performed using priori linear contrasts or posteriori Tukey tests. To identify intergroup differences in survival functions, the Gehan generalised Wilcoxon test was used. The frequency of alternative traits occurrence was determined by the Fisher exact test. The critical significance level of a was assumed to be 0.05.
RESULTS
In the first series of experiments, 2–3 min after a single intranasal instillation of PNS 170 mg/mL 10 µL, the motor activity of the rats decreased; the animals looked inhibited. The results of studying the effect of PNS on oxygen consumption and external respiration are shown in Fig. 1.
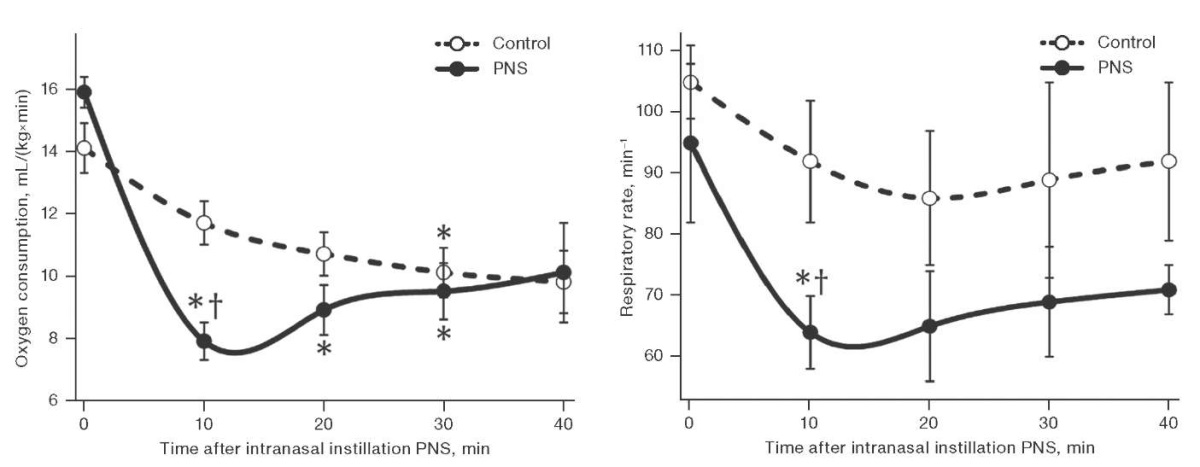
Figure prepared by the authors based on their own data
Fig. 1. Dynamics of oxygen consumption (left) and respiratory rate (right) in male rats after a single intranasal instillation of PNS
Note: the data is presented as mean and the standard error of the mean (M ± m); * — statistically significant difference from the baseline; † — statistically significant difference from the control group.
Figure 1 shows that 10 min after the use of PNS, oxygen consumption by animals of the experimental group decreased by 33%, and the respiratory rate decreased by 32% compared to the control group. The gas exchange efficiency of external respiration, estimated by oxygen consumption per respiratory cycle, did not change significantly. In the following 20 min, oxygen consumption in rats treated intranasally with a single dose of PNS remained 9–20% lower than in the control, although levelling out. Thus, the duration of the hypometabolic effect of a single PNS instillation was at least half an hour.
The second series of experiments, when studying the effect of PNS 170 mg/mL in a volume of 10 µL on the state of thermal metabolism in rats, found that animal staying in a thermal chamber led to a statistically significant increase in their rectal temperature. In animals placed in restrainers, the temperature increased by 5.5 ± 0.3°C within an hour versus 3.3 ± 0.3°C in animals freely housed in enclosures. At the same time, the use of PNS accelerated the increase in body temperature by 15% in rats housed in enclosures, although slowing it down by 11% in those kept in restrainers (Fig. 2a). When placed in enclosures, the animals that received saline solution showed active grooming, which was not the case with those receiving PNS. About 120 min after being placed in enclosures, the body loss of moisture estimated by weight loss due to the use of PNS was 1% less of the initial body weight than in the control (Fig. 2b). The life expectancy of rats in enclosures was longer than in restrainers. When placed in enclosures, the use of PNS reduced life expectancy; when placed in restrainers, life expectancy tended to increase (Fig. 2c).
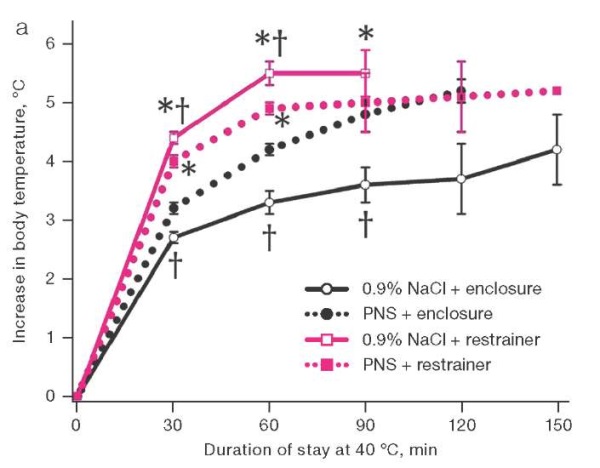
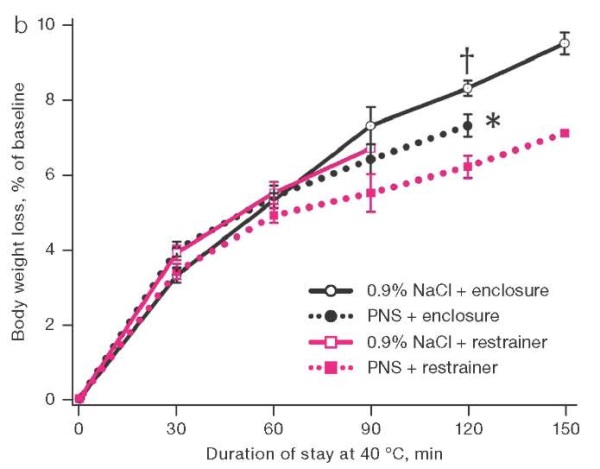
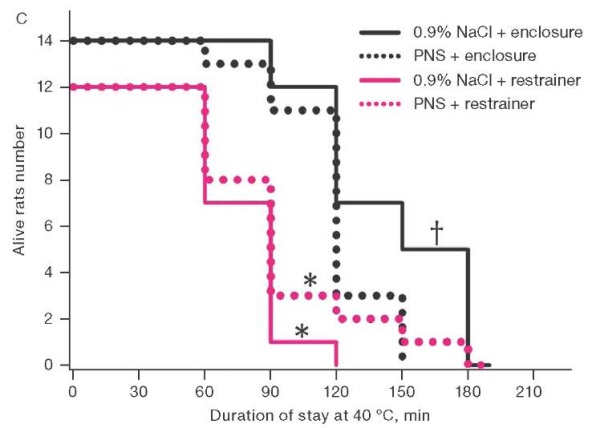
Figure prepared by the authors based on their own data
Fig. 2. Dynamics of rectal temperature, body weight loss, and survival in rats at an air temperature of 40°C.
Note: the increase in rectal temperature and body weight loss are presented as mean and the standard error of the mean (M ± m); * — statistically significant difference from the group of rats housed in the enclosure; † — statistically significant difference from the group of rats treated with PNS under similar placement conditions.
DISCUSSION
With a single instillation of 10 µL of PNS, the phenazepam dose for rats was 8.5 mg/kg bw, which is bioequivalent to 100 mg for an adult human. This was five times higher than the highest single intravenous dose of diazepam 20 mg, which reduced human oxygen consumption by 8% [9]. Therefore, the fourfold greater hypometabolic effect of PNS observed in this study was due to the benzodiazepine dose, which, when extrapolated to humans, was five times higher than the highest single dose. The duration of this effect was close to half an hour, which indicates the need for repeated PNS prescriptions to achieve a longer hypometabolic effect.
As follows from the increase in body temperature, staying in a heat chamber caused thermal stress in the rats. Due to the impossibility of grooming, the distribution of moisture (presumably saliva) over the coat, and, therefore, the loss of heat by evaporation, was hampered in rats placed in restrainers. This made the heat transfer conditions comparable for animals placed in restrainers that received intranasal instillations of PNS or a sodium chloride solution. Therefore, the probable reason for the decrease in thermal stress in rats housed in restrainers, in the setting of PNS, was a decrease in heat production, rather than an increase in heat transfer.
The decrease in heat production in the animals that received PNS before being placed in a thermal chamber was assessed using the caloric equivalent of oxygen, which is close to 21 J/mL in the case of a carbohydrate-dominated diet [10]. During 30 min after PNS instillation, oxygen consumption by animals was on average 2.06 mL/(kg×min) lower than in the control, which corresponds to 1294 J/kg of lower heat production. When the specific heat of biological tissues and water are close to 4187 cal/(kg×°C), this could decrease by 0.3°C the accumulation of body temperature over the following 30 minutes after placing the rats in a thermal chamber, which was observed in the present work. Thus, when the air temperature was elevated and moisture could not evaporate from most of the body surface, the introduction of PNS slowed down the formation of thermal stress in rats, reducing heat production. In order to extrapolate these data to humans, it seems relevant to study the effect of PNS on body temperature under the conditions that prevent heat transfer by evaporation, i.e., at high relative humidity [11], exposure to insulating skin protectors [12], or with immersion hyperthermia [13]. The ability of diazepam, administered intravenously in doses of 10 or 20 mg, to reduce human body temperature [14] allows us to expect its decrease even when using PNS.
An optimal relative humidity and a sufficient rate of air exchange in the thermal chamber favored heat loss by evaporation of moisture by rats who had the opportunity to apply it to the surface of the body. Therefore, when they were placed in aviaries, body temperature rose more slowly than that in restrainers. However, the introduction of PNS smoothed out this difference: the medicine accelerated the increase in body temperature. Following 120 min of being placed in enclosures, body weight loss, mainly due to evaporation of water, was 10 g/kg less with PNS instillation than with the introduction of saline solution. Under the specific heat of water vaporization of 2260 J/g, this corresponded to the accumulation of 22,600 J/kg more heat in the body of animals than in the control. Under the specific heat capacity of biological tissues close to 4187 J/(kg×°C), this energy was sufficient to increase body temperature by 5.4°C. In fact, in rats that received PNS, it was only 2°C higher than in the control. This indicates the influence of oppositely directed factors on the body temperature of rats who received PNS, including a decrease in heat transfer and the decrease in heat production shown above. As can be seen, in animals that had no physical obstacles to moistening the body surface, the effect of PNS on heat transfer prevailed over its effect on heat production.
Adverse pharmacological effects of benzodiazepines could play a certain role in reducing the survival rate of rats that received PNS before being freely placed in a thermal chamber, including an uncoupling effect on oxidative phosphorylation [15], reduction of blood pressure, heart rate and coronary blood flow [16]. However, the main mechanism of the PNS-induced decrease in survival in conditions of free location in a thermal chamber was the aggravation of thermal stress. This is indicated by the fact that 90 min after being placed in a thermal chamber, the body temperature of 43°C, corresponding to the threshold of irreversible damage to biological tissues [17], was reached in 21% and 71% of animals treated with saline solution and PNS, respectively (p < 0.05). Extrapolation of these data to humans is difficult due to the presence of sweat glands on the entire surface of the skin, which ensures heat transfer by evaporation even against the background of physical inactivity. At the same time, impaired heat transfer is not excluded in humans due to reports of antagonism of benzodiazepines to muscarinic receptors [18], which requires consideration of climatic conditions when developing recommendations for the use of PNS.
The results obtained indicate the possibility of mitigating thermal stress with the use of PNS at high relative humidity, exposure to insulating skin protectors, or immersion hyperthermia. Such an opportunity becomes particularly valuable with continued heat exposure or the unavailability of physical means of cooling the body at the pre-hospital stage of assistance to victims.
CONCLUSIONS
- A single intranasal instillation of phenazepam in rats at a dose of 8.5 mg/kg bw, which is bioequivalent to 100 mg for an adult human, reduced the body oxygen consumption by an amount sufficient to reduce the 0.3°C increase in body temperature over 30 min of exposure to an air temperature of 40°C. The duration of the hypometabolic effect of a single instillation of the nasal preparation of phenazepam (PNS) was at least half an hour, which ensured this effect in animals receiving the drug with a half-hour interval.
- At an air temperature of 40°C and in the absence of conditions for evaporation of moisture from the body surface, the administration of PNS to rats reduced heat stress, decreasing heat production and increasing rectal temperature by 0.3°C in 30 min.
- The open arrangement of rats at an air temperature of 40°C in enclosures, which did not create obstacles to heat transfer by evaporation of saliva applied to the coat, was accompanied by a slower increase in body temperature and a lower mortality than when animals were placed in restrainers.
- The instillation of PNS contributed to an increase in body temperature and mortality in rats housed in enclosures at an air temperature of 40°C. The main mechanism of these effects was a decrease in heat transfer by evaporation due to the suppression by PNS of a species-specific thermoregulatory reflex, i.e., application of saliva to the coat.
- PNS is a promising means of pharmacotherapy for heat stroke at high relative humidity, exposure to insulating skin protectors, or immersion hyperthermia. The possibility of the aggravating effect of PNS on human thermal stress in a hot climate with unhindered heat transfer by evaporation needs to be verified.
1. Order of the Ministry of Health of the Russian Federation No. 1115n. Standard of emergency medical care for heat and sunstroke; 12/20/2012.
References
1. Cramer MN, Gagnon D, Laitano O, Crandall C. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022;102(4):1907–89.https://doi.org/10.1152/physrev.00047.2021
2. Garcia CK, Renteria LI, Leite-Santos G, Leon LR, Laitano O. Exertional heat stroke: pathophysiology and risk factors. BMJ Ved. 2022;1(1):e000239. https://doi.org/10.1136/bmjmed-2022-000239
3. Крюков ЕВ, ред. Военно-полевая терапия. СПб.: ООО «ГЭОТАР-Медиа 2023.
4. Краснов КА, Ивницкий ЮЮ, Вакуненкова ОА, Рейнюк ВЛ. Фармацевтическая композиция для устранения судорожного синдрома на основе никетамида и препаратов бензодиазепиновой группы. Патент Российской Федерации № 2801050;2023.
5. Ивницкий ЮЮ, Рейнюк ВЛ, Краснов КА, Краснова АА, Шутаева КВ. Требования к медикаментозным средствам первой помощи при судорожном синдроме химической этиологии (обзор литературы). Воен. мед. журн. 2022;343(3):18–23. https://doi.org/10.52424/00269050_2022_343_3_18
6. Fleiscer JE, Milde JH, Moyer TP, Michenfelder JD. Cerebral effects of high-dose midazolam and subsequent reversal with Ro 14-1788 in dogs. Anesthesiol. 1988;68(2):234–42. https://doi.org/10.1097/00000542-198802000-00010
7. Hostler D, Northington WE, Callaway CW. High-dose diazepam facilitates core cooling during cold saline infusion in healthy volunteers. Appl. Physiol. Nutr. Metab.2009;4(4):582–6. https://doi.org/10.1139/H09-011
8. Attah AT, Negrón-Moreno PN, Amigo-Duran M, Zhang L, Kenngott M, Brecht M. et al. Sensory cues, behavior and fur-based drying in the rat wetness response. Sci Rep. 2024;14(1):24550. https://doi.org/10.1038/s41598-024-74900-9
9. Griffe O, Griffe H, du Cailar J. Effects of diazepam on oxygen consumption. Ann. Aneshesiol. Fr. 1979;20(1):37–40.
10. Симбирцев ГС. Регулирующее влияние углекислого газа на потребление кислорода у спортсменов, развивающих выносливость, в свете математического анализа продукции энергии аэробного окисления. Спортивная медицина: наука и практика. 2019;9(3):12–24. https://doi.org/10.17238/ISSN2223-2524.2019.3.12
11. Wolf ST, Bernard TE, Kenney WL. Heat exposure limits for young unacclimatized males and females at low and high humidity. J. Occup. Environ. Hyg. 2022;19(7):415–24. https://doi.org/10.1080/15459624.2022.2076859
12. Zhao Y, Su M, Meng X, Liu J, Wang F. Thermophysiological and perceptual responses of amateur healthcare workers: impacts of ambient condition, inner-garment insulation and personal cooling strategy. Int. J. Environ. Res. Public Health. 2022;20(1):612. https://doi.org/10.3390/ijerph20010612
13. Ким АЕ, Шустов ЕБ, Зайцева ИП, Лемещенко АВ. Патофизиологические механизмы неблагоприятного взаимодействия гипоксии и температурных факторов в отношении физической работоспособности. Пат. Физиол. Эксперим. Терап. 2022;66(4):94–106. https://doi.org/0.25557/0031-2991.2022.04.94-106
14. Hostler D, Northington WE, Callaway CW. High-dose diazepam facilitates core cooling during cold saline infusion in healthy volunteers. Appl. Physiol.Nutr. Metab. 2009.34(4):582–86. https://doi.org/10.1139/H09-011
15. Jeso F, Truscello A, Martinotti G, di Jeso B, Magnani B, Martinotti A. Effect of diazepam on mitochondrial respiration. C. R. Seances. Soc. Biol. Fil. 1990;184(1):37–40.
16. Shintani R, Ichinomiya T, Tashiro K, Miyazaki Y, Tanaka T, Kaneko S, et al. Comparison of hemodynamic effects of remimazolam and midazolam during anesthesia induction in patiemts undergoing cardiovascular surgery: a single-center retrospective and exploratory study. Cureus. 2024;16(10):e72032. https://doi.org/10.7759/cureus.72032
17. Yarmolenko PS, Moon EJ, London C, Manzoor A, Hochman DW, Viglianti L, Dewhirst MW. Thresholds for thermal damage to normal tissues: an update. Int. J. Hyperthermia. 2011;27(4):320–43. https://doi.org/10.3109/02656736.2010.534527
18. Hiroshi K, Naoko T, Mitsuru K, Mostofa J, Asuka I, Nagata K, et al. An autopsy case of heatstroke under the influence of psychotropic drugs. Soud. Lek. 2020;65(4):76–8.
About the Authors
Ju. Ju. IvnitskyRussian Federation
Jury Ju. Ivnitsky
St. Petersburg
O. A. Vakunenkova
Russian Federation
Olga A. Vakunenkova
St. Petersburg
K. A. Krasnov
Russian Federation
Konstantyn A. Krasnov
St. Petersburg
S. S. Gaft
Russian Federation
Semion S. Gaft
St. Petersburg
N. V. Lapina
Russian Federation
Natalja V. Lapina
St. Petersburg
Review
For citations:
Ivnitsky J.J., Vakunenkova O.A., Krasnov K.A., Gaft S.S., Lapina N.V. Heat transfer by evaporation determines the effect of nasal phenazepam on thermal stress in rats. . 2025;27(1):37-42. https://doi.org/10.47183/mes.2024-245





