Scroll to:
Luminescent immunochromatography based on Eu³⁺ coordination compounds for detection of pathogenic microorganisms and bacterial toxins
https://doi.org/10.47183/mes.2025-257
Abstract
Introduction. Increasing the sensitivity of immunochromatographic assay as an express method for detection and diagnosis of pathogenic microorganisms and toxins is a relevant task with regard to ensuring food safety and population health in general.
Objective. Development of a prototype of a video digital recorder of luminescent immunochromatograms (RLI-Eu³⁺) and luminescent immunochromatographic tests (LICHT) adapted to a device based on microspheres with europium compounds. Comparison of the sensitivity of LICHT and AuNPs-based immunochromatography assay (ICA) tests to increase the sensitivity of the method for detection of pathogenic microorganisms and their toxins.
Material and methods. Submicron polymer microspheres labeled with organic complexes of trivalent europium (Eu³⁺) were used as a luminescent label for immunochromatography. LICHT were recorded using the developed RLI-Eu³⁺ recorder.
Results. The detection threshold of the Eu³⁺ luminescent complex on the immunochromatographic membrane was shown to be 2 pg/mm², with the linearity of the readings ranging within 2–200 pg/mm². The coefficients of variation of the instrument readings in the luminescent complex concentration range of 20–200 pg/mm² and 2–20 pg/mm² were found to be less than 5% and 10%, respectively. Using LICHT and the RLI-Eu³⁺ device, detection thresholds were determined as follows: cholera toxin — 10 ng/mL, staphylococcal enterotoxin type B — 0.5 ng/mL, plague pathogen cells — 1×10³ cells/mL, anthrax pathogen spores — 5×10³ spores/mL, Crimean-Congo hemorrhagic fever (CCHF) virus antigens — at a dilution of 1:640,000.
Conclusions. The developed video digital recorder RLI-Eu³⁺ and LICHT based on europium coordination compounds enabled the detection threshold of pathogenic microorganisms and bacterial toxins to be reduced by 20–128 times compared to immunochromatographic tests based on colloidal gold (CG) for the same pathogens.
Keywords
For citations:
Yarkov S.P., Tretyakov S.I., Shilenko I.V., Shaulina E.K., Mandaji A., Zenkov D.A., Ishkov Yu.N., Styazhkin K.K. Luminescent immunochromatography based on Eu³⁺ coordination compounds for detection of pathogenic microorganisms and bacterial toxins. Extreme Medicine. 2025;27(1):107-114. https://doi.org/10.47183/mes.2025-257
INTRODUCTION
Immunochromatographic assay (ICA) in its various schemes is widely used as a rapid clinical and laboratory diagnostic tool for detecting pathogenic microorganisms and toxins in the fields of, inter alia, food safety and environmental monitoring.
Luminescent immunochromatographic assay (LICA) is a versatile rapid diagnostic method (e.g., for detection of specific antibodies to the foot-and-mouth disease virus in blood serum [1]), in which luminescent particles are used to label antibodies. LICA quantifies the blood level of cardiac markers by analyzing the luminescence intensity of a test strip [2][3]. The advantages of this method include its high reproducibility, sensitivity, and noise immunity against the natural fluorescence of the test sample. LICA is widely used for ensuring food safety [4][5] and environmental monitoring [6].
In comparison with conventional immunochromatography labels — colloidal gold nanoparticles (AuNPs), LICA provides a two- or threefold increase in sensitivity [7][8]. A comparison of fluorescent markers with AuNPs-based labels and magnetic nanoparticles in ICA can be found in [9]. Among the luminescent markers used in LICA, polymer microspheres with a diameter of 0.1–0.3 µm stand out. Their surface contains carboxyl groups, which allows covalent bonding with receptor proteins, e.g., immunoglobulins of various classes. When obtaining microspheres during polymerization, organic complexes of trivalent europium are introduced into the reaction environment, such as Eu(DBM)3Phen, (1,10-phenanthroline)-tri- (dibenzoylmethanate) europium; Eu(TTA)3Phen, (2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline-tri-tenoyl triphluoroacetonate) europium in the amount of no greater than 10 wt % [10]. Preparations of such microspheres absorb light in the near ultraviolet band at λmax = 365 nm and fluoresce in the red region at λmax = 615 nm, while possessing a narrow emission peak of fluorescence. Semiconductor diodes, photoelectronic multipliers, and video cameras are used to register the luminescence of the analytical and control zones of immunochromatography (AZ, CZ). The digital video recording (DVR) method of ICA results with various labels has a number of advantages over other design solutions. Thus, the DVR of AuNPs-based tests makes it possible to obtain quantitative data on the content of toxins in dairy products [11].
In this study, we aim to develop a prototype of a video digital recorder for luminescent immunochromatography RLI–Eu3+ and luminescent immunochromatographic tests (LICHT) adapted to the device based on microspheres with europium compounds. A comparison of the sensitivity of LICHT and AuNPs-based ICA tests is conducted with the purpose of increasing the sensitivity of the method in identifying pathogenic microorganisms and their toxins.
MATERIALS AND METHODS
In order to obtain conjugates, we used monoclonal antibodies MAB to the B-subunit of the cholera toxin (CT), clone 3D11 (HyTest, Russia); MAB to capsular F1 antigen of the plague pathogen; MAB to anthrax spores, clone SA26; MAB to SEB (staphylococcal enterotoxin В) clone S222 (VNCMDL, Russia); mouse polyclonal immunoglobulins to CCHF (Gamaleya National Research Center for Epidemiology and Microbiology). The same batches of antibody preparations were used to create LICHT and AuNPs-based tests.
The certified CT and SEB preparations, inactivated Y. pestis microbial cells (strain EV), inactivated B. anthracis cells, strain 942 (State Research Center for Applied Microbiology and Biotechnology), certified sucrose-acetone antigens of the CCHF virus (strain 741), genotype Europe 1 (Gamaleya National Research Center for Epidemiology and Microbiology) were used as analyzed antigens.
The synthesis of antibody conjugates with luminescent microspheres was carried out by covalent binding [12]. 5 µL of suspension of luminescent microspheres (1%, weight/volume) were dispersed in 1.0 mL of MES buffer (0.05 M, pH 6.0) and supplemented with 10 µL EDC and NHS solutions (0.5 mg/mL) to activate the carboxyl groups contained on their surface. The resulting suspension was stirred and shaken on a Vortex-3000 laboratory vortex shaker (Viggens, China) for 20 min at room temperature in the dark followed by centrifugation on a Thermo Scientific SL 4R Plus centrifuge (Thermo Fisher Scientific, USA) at ×9600 g for 15 min; the supernatant was discarded. The precipitate was resuspended in 1.0 mL of phosphate buffer (0.01 M, pH 7.4). Then 100 µL of an antibody solution was added at a concentration of 0.2 mg/mL. The resulting mixture of antibodies and activated luminescent microspheres was continuously stirred for 2 h at room temperature in the dark. Subsequently, 100 µL of a blocking solution (20% bovine serum albumin BSA) was added to block non-specific binding sites on the surface of the luminescent microspheres; the mixture was kept at room temperature for 1 h. The resulting suspension was centrifuged at 8000 g for 15 min, the supernatant was discarded, and 200 µL of a dispersing solution was added to the precipitate to prevent agglutination of conjugates of luminescent microspheres with antibodies (0.02 M, TRIS-HCl) containing 0.5% (weight/volume) trehalose, 10% (weight/volume) sucrose, 0.5% polyvinylpyrrolidone, 0.1% Tetronic 1307 (S9), 0.05% ProClinTM 300, 1% BSA and 0.1% Tween-20). Prior to application, the solution was stored at 4 °C.
For the formation of the LICHT analytical zone, the following antibodies were used: rabbit polyclonal antibodies to the B-subunit of the cholera toxin; MAB to the capsular F1 antigen of the plague pathogen clone F41F8C9; MAB to anthrax spores clone 278H4A7 (branch of the 48th Central Research Institute of the Russian Ministry of Defense, Kirov); mouse immunoglobulins to CCHF antigens (Gamaleya National Research Center for Epidemiology and Microbiology); MAB to SEB S643 (VNCMDL, Russia) at a concentration of 2.0 mg/mL. The antibodies of the LICHT control zone were goat antibodies to mouse immunoglobulins and goat antibodies to rabbit immunoglobulins (HyTest) at a concentration of 0.1 mg/mL.
A GFCP001000 glass fiber membrane and CFSP203000 and CFSP173000 cellulose membranes (Millipore), cards with TYPE-CNPF-SN12-L2-P25, 10 µ (Mdi, India) nitrocellulose membrane were used to manufacture the LICHT multimembrane composite. The finished test strips were placed in TYPE-Device-1 plastic frames (Mdi, India), the top cover of which was coated with a matte black paint. Prior to application to the glass fiber membrane, the conjugate of microspheres with antibodies was treated with ultrasound on a Labsonic 2000 dispersant (B. Braun, Germany) for 10 s followed by stirring. Then, a storage buffer was added to a concentration of 0.01%. The resulting conjugate was manually applied onto a GFCP001000 (Millipore) fiberglass membrane and dried at room temperature.
A video digital luminescent immunochromatographic analysis was performed using an RLI-Eu3+ device at room temperature for 25 min after the introduction of the analyte. The volume of the analyte introduced into the LICHT was 140 µL.
Control samples (CS) intended for studying the analytical characteristics of the recording device were produced in two versions, differing in the range of concentrations of luminescent microspheres in the analytical zone. CSs were immunochromatographic membranes on which a suspension of luminescent microspheres with europium with a linear density of 0.08 µL/mm was applied in strips using an IsoFlowTM dispenser (Imagene Technology Inc, USA). The membranes were dried at room temperature, cut into strips 4 mm wide using a Matrix 2360 programmable guillotine (Kinematic Automation Inc, USA), and placed in rims for multianalytical immunochromatographic elements. A suspension of microspheres at a concentration of 0.01% was used to form a short circuit. Concentrations of microspheres from 0.00005% to 0.01% were used to form the AZ. The area of each zone on the membrane with luminescent microspheres was 4 mm2. In this way, an imitation of LICHT with different luminescence intensities was obtained. CSs were stored in light-proof bags at 4 °C. The following reagents were used in the study: 1% (weight/volume) suspension of carboxylated polystyrene microspheres with a europium complex in deionized water with the addition of 0.05% NaN3 (Bang Laboratories, USA). The average diameter of microspheres was 190 nm. 2-(N-morphino)-ethanesulfonic acid (MES) monohydrate, tris-(hydroxymethylaminomethane) hydrochloric acid (TRIS-HCl) salt, Twen-20 bovine serum albumin (BSA) free of proteases, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) hydrochloride, N-hydroxysuccinimide (NHS), sodium hydroxide, ProClinTM-300, glycine, polyvinylpyrrolidone MM 40,000, sodium chloride — all these materials were produced by Sigma-Aldrich. Trehalose-D(+) — dihydrate for biochemistry, hydrochloric acid (chemically pure, C.P.) (Dia-M (Russia), Tetronic 1307(S9), Braveds (China) were used.
Particles with the average diameter of 30 nm were used as a conjugate label for the manufacture of AuNPs-based immunochromatographic tests [11]. The immune components of such tests were identical to those used in LICHT. Multimembrane composites and test manufacturing techniques were similar to LICHT manufacturing techniques. A video digital immunochromatographic Reflex analyzer (Registration certificate for a medical device dated April 26, 2016 No. FSR 2011/11281/, TU9443-001-43312649-2014, Sinteko-Complex, Russia) was used as a digital video recorder (DVR).
The results were processed using the Microsoft Excel 2003 program, the Descriptive Statistics option.
RESULTS
The developed prototype of a video digital recorder of luminescent immunochromatograms RLI-Eu3+ consists of a measuring unit and a laptop with a pre-installed software application for monitoring and calculating the obtained results. The appearance of the device is shown in Fig. 1.
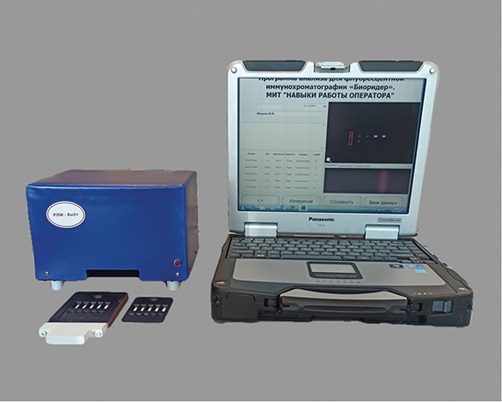
Figure prepared by the author using their own data
Fig. 1. Appearance of the developed luminescent immunochromatography recorder RLI-Eu3+
The measuring unit contains a fluorescent light source consisting of two 4 W mercury discharge lamps. The lamp flasks are made of Wood glass, which creates a field of uniform ultraviolet irradiation of immunochromatograms with a maximum wavelength at 365 nm, as well as a lamp power source. A solid-state video camera with a resolution of 1200×1600 pixels and a video shooting speed of 15 frames per sec is also located in the housing of the measuring unit. A SV580 glass luminescence emission filter is installed in front of the camera lens, ensuring a 90% transmission of radiation in the wavelength range from 600 nm to 900 nm. The video camera is connected to a laptop via a USB 2.0 connector. Plastic frames containing LICHT membranes are placed inside the device using a holder. The device allows simultaneous registration of up to five immunochromatograms; however, the possibility of measurement and single tests is also provided.
The images obtained using the device video camera were processed by a fluorescent immunochromatogram analysis software application running in Windows 7, 10.
The device provides calculation of the integral intensity of the LICHT analytical and control zones with automatic correction of the baseline. The criterion for a positive result of DVR analysis was the excess of the luminescence intensity of the analytical zone of the test over the average background value in an idle experiment, taking into account the measurement error with a 95% confidence probability:
[Хmean — ts × SE]signal ≥ [Хmean + ts × SE]background, (1)
where Xmean — mean of n-measurements;
ts — Student’s coefficient for n-measurements;
SE — standard error mean at 95% confidence level.
The RLI-Eu3+ luminescence recorder has the following technical characteristics: weight — 1.64 kg, dimensions — 150×200×170 mm, power supply — 220–240 V, 0.15 A, 50 Hz. Management from an external laptop with Windows 7.10 OS is performed using a specialized software application. The maximum wavelength of exciting light is λex = 365 nm; the maximum wavelength of emission is λem = 615 nm. The detection limit of the luminescent substance on the immunochromatographic membrane is 2.0 pg/mm2. The number of immunochromatograms in the test frame can vary from one to five. The coefficient of variation of the luminescence intensity readings of the analytical zone of the test in the concentration range of the luminescent complex is 2–20 pg/mm2 <10%, in the range of 20–200 pg/mm2 <5%. The linearity of readings ranges within 2–200 ng/mm2. The operating temperature ranges from 5 °C to 35 °C.
Table 1 shows the results of CS measurements using the RLI-Eu3+ instrument in various concentration ranges of the luminescent europium complex in the CS analytical zone.
Table 1. Luminescence intensity in the CS analytical zone as a function of the concentration of the luminescent europium complex on the membrane
|
Concentration of microsphere suspension during the formation of the analytical zone, % |
1×10–2 |
5×10–3 |
2.5×10–3 |
1×10–3 |
5×10–4 |
2.5×10–4 |
1×10–4 |
5×10–5 |
0 |
|
Concentration of the luminescent europium complex on the membrane, pg/mm2 |
200 |
100 |
50 |
20 |
10 |
5 |
2 |
1 |
0 |
|
Average luminescence intensity, conl. units. |
552.3 |
314 |
144.3 |
45.5 |
44.3 |
24.4 |
12.1 |
3.5 |
1.06 |
|
Standard deviation (SD) |
13.0 |
12.1 |
5.9 |
2.3 |
3.9 |
2.0 |
1.2 |
1.7 |
0.62 |
|
Coefficient of variation (CV), % |
2.35 |
3.9 |
4.09 |
5.05 |
8.80 |
8.20 |
9.92 |
48.57 |
58.49 |
|
Standard error (SE) |
4.1 |
4.0 |
1.9 |
0.7 |
1.2 |
0.6 |
0.4 |
0.5 |
0.20 |
|
Confidence interval (95.0%) |
9.3 |
9.3 |
4.2 |
1.7 |
2.8 |
1.4 |
0.9 |
1.2 |
0.44 |
Table prepared by the authors based on their own data
Table 1 demonstrates satisfactory values of the coefficient of measurement variation (CV) in the range of microsphere concentrations from 20 pg/mm to 200 pg/mm2, which indicates a high reproducibility of measurement results using the RLI-Eu3+ device. Higher values of the coefficient of variation are observed at measuring concentrations <5 pg/mm2. The detection sensitivity of luminescent microspheres on the membrane surface reaches 1 pg/mm2, which is 3.5 times higher than the average luminescence values in an idle experiment.
The linearity of instrument readings during measurement is shown in Fig. 2.
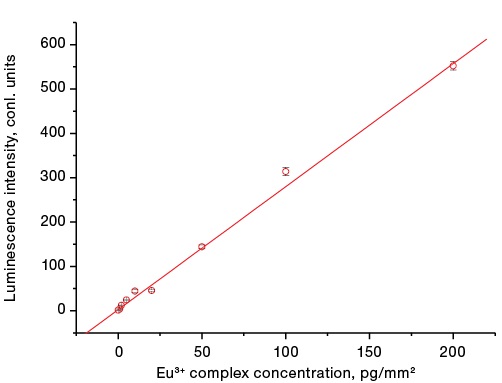
Figure prepared by the author using their own data
Fig. 2. Dependence of the luminescence intensity of the CS analytical zone on the surface concentration of the europium complex on the analytical membrane
A linear regression for the dependence of the luminescence intensity of microspheres on the membrane on the microsphere concentration was constructed using the least squares method using the Origine 6.1 software (OriginLab Corp.). The correlation coefficient of the linear relationship is R = 0.99.
Luminescent tests for the immunochromatographic detection of pathogenic microorganisms and toxins were developed in a sandwich format. A detailed description of AuNPs-based immunochromatographic tests in the sandwich format and the analytical procedure for detecting bacterial toxins and spore forms of microorganisms can be found in [13][14].
During the study, a liquid sample potentially containing pathogenic microorganisms or toxins was applied onto a sample substrate. Under the action of capillary forces, liquid moves through the multimembrane composite. First, the conjugate of microspheres with specific immunoglobulins immobilized on the surface is solubilized. The conjugate of microspheres is luminesced in the red region of the spectrum. In the presence of a detectable antigen in the sample, an antigenic immune complex is formed. This complex moves along the analytical membrane with an excess of conjugate with the flow of liquid. Next, the immune complex is captured on the analytical membrane by specific antibodies in the AZ, forming a sandwich. The unbound conjugate antibodies are captured by antibodies in the CZ of the test strip, which leads to the formation of two luminescent lines. In the absence of an antigen in the sample, an antigenic immune complex is not formed; therefore, the only line visible in ultraviolet light is formed by binding conjugate antibodies and CZ antibodies (anti-specific with respect to conjugate antibodies) only in the CZ. The appearance of tests for detecting cells of the vaccine strain of the plague causative agent based on AuNPs and on the basis of luminescent microspheres under ultraviolet irradiation with a wavelength of 365 nm is shown in Fig. 3.
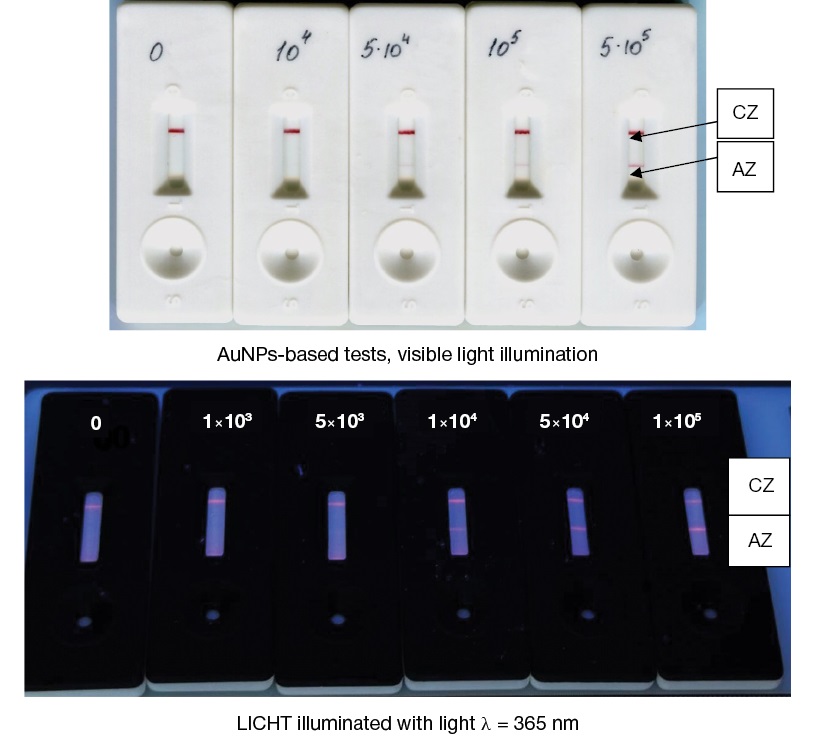
Figure prepared by the authors based on their own data
Fig. 3. Appearance of immunochromatograms of Y. pestis EV cells.
Note: Arrows and letters indicate the analytical (AZ) and control (CZ) test zones. Concentrations are indicated in cells/mL.
Table 2 shows the detection (LICHT and AuNPs-based immunochromatography) thresholds for various pathogenic microorganisms and bacterial toxins.
Table 2. Comparative characteristics of LICHT and immunochromatographic tests based on AuNPs in the detection of toxins and pathogenic microorganisms
|
Analyte |
Ratio of the minimum detectable analyte concentrations for different conjugate labels of immunochromatographic tests |
||
|
LICHT based on microspheres with Eu3+complex, luminescence DVR |
ICA based on the AuNPs, reflected light DVR |
Ratio of LICHT sensitivity to ICA sensitivity |
|
|
Staphylococcal enterotoxin type B, ng/mL |
0.5 |
30 |
60 |
|
Cholera toxin, ng/mL |
10 |
500 |
50 |
|
Microbial cells of Y. pestis, strain EV, mg/mL |
1х103 |
5х104 |
50 |
|
B. anthracis spores/mL |
5х103 |
1х105 |
20 |
|
Causative agent of CCHF (dilution of the initial preparation of viral antigens) |
1:640,000 |
1:5000 |
128 |
Table compiled by the authors based on their own data
Using the example of microorganisms of various taxonomic groups (vegetative and spore forms of bacteria, viruses, and bacterial toxins), Table 2 shows that the use of immunochromatography with conjugates based on microspheres with luminescent labeling of europium compounds provides a much greater sensitivity than the use of tests with AuNPs conjugates. The correctness of the comparison is ensured by using the same batches of antibodies to produce tests based on luminescent antibodies and tests based on colloidal gold nanoparticles. When comparing the sensitivity level, identical antigenic drugs were used.
DISCUSSION
The use of digital video recording of immunochromatograms in combination with microspheres containing europium complexes provides a significant gain in ICA sensitivity. This is due to the significant volume of microspheres containing a large number of europium complexes per particle and the photophysical properties of the europium complexes themselves. These compounds are excited by UV light in the near range of 365–370 nm and emit luminescence in the red region.
The significant Stokes shift is due to the processes of excitation of the chelated ligand of the complex and further energy transfer to the 5D0 energy level of the Eu3+ ion with subsequent transitions between the 5D0 → 7F2 level and light emission in the range of λ = 610–660 nm with a high quantum yield. Practically speaking, this makes it possible to use inexpensive glass filters in the device design to isolate exciting light and emission light, which provides a good contrast between the lines of immunochromatograms and the background. Another advantage of such labels is the small width (<10 nm) of the emission lines and the long luminescence lifetime of microspheres with europium t = 0.43–0.55 ms. The europium luminescent complex isolated in a polystyrene matrix is stable, being protected from exposure to atmospheric oxygen and luminescence-extinguishing substances potentially present in the analyte and buffer solutions used in ICA. However, these compounds are not devoid of drawbacks, since they reduce the quantum yield of luminescence with an increase in temperature. Thus, europium complexes polymerized in films have stable luminescence in the range from –60 °C to +25 °C. When the film is heated to a temperature of above +25 °C, a decrease in the intensity and decay time of luminescence corresponding to the 5D0 → 7F2 transition is observed [15]. The effect is reversible; thus, upon cooling, the luminescence intensity is restored. This phenomenon can reduce the sensitivity of LICHT when used in field studies at elevated temperatures.
According to the passport data of the drug, the surface of one microsphere contains up to 353,000 of carboxyl groups with a microsphere average diameter of 190 nm. This allows for effective conjugation of luminescent microspheres with antibody proteins due to the formation of covalent bonds between the amino groups of antibodies and the carboxyl groups of microspheres. The conjugate of microspheres with immunoglobulins has a significant number of antibody valences, which contributes to the effective formation of immune complexes with antigens of bacteria, toxins, and viruses [16].
The use of video cameras as a radiation receiver is also a significant advantage in DVR [17], since the latter have high sensitivity and the ability to change the brightness, contrast, and gamut of the image. These properties of video cameras make it possible to improve the image of immunochromatograms in a wide range of luminescence intensity. In addition, the DVR does not require mechanical drives (actuators) to move immunochromatograms under the beam of exciting light.
We have obtained data on significant differences in the sensitivity of LICHT and AuNPs-based immunochromatographic tests in the detection of toxins and pathogenic microorganisms. The increased sensitivity of detection of microorganisms and toxins in the use of fluorescent tags compared to tests based on AuNPs is primarily due to the higher sensitivity of detection of fluorescent tags compared to the sensitivity of detection of colorimetric tags (AuNPs). An important factor in increasing sensitivity is the greater number of capture antibodies in the immunochromatographic conjugates of luminescent microspheres compared with similar conjugates of AuNPs. This is due to the geometric dimensions of the microparticles: the AuNPs average diameter is 30 nm, compared to the luminescent microspheres average diameter of 190 nm. These factors lead to a more efficient process of immunochromatography and detection results. It should be noted that the preparation of samples for analysis, the procedure for the analysis, and the time of its implementation do not change. Technologically, the production of LICHT and ICA tests based on AuNPs are also similar, with the exception of the conjugate synthesis stage. Therefore, no changes in the technological line of production equipment are required.
The high sensitivity of LICHT is important for the sanitary and hygienic aspect of the use of immunochromatography in general, making it possible to detect lower concentrations of pathogenic microorganisms and toxins in environmental objects, food products, and body fluids. LICHT can be useful for the immunochemical verification of microbial crops using the conventional microbiological method by reducing the cultivation time, allowing the identification of cells and bacterial toxins across a shorter period of time.
CONCLUSIONS
- An experimental prototype of a video digital recorder of luminescent immunochromatograms RLI-Eu3+ has been developed, where submicron microspheres containing complex compounds of trivalent europium conjugated with specific antibodies are used as a label for immunochromatography.
- RLI-Eu3+ has a sensitivity threshold for the luminescent europium complex of 2 pg of the substance per 1 mm2 of the immunochromatographic membrane and a linearity in the range of 2–200 pg/mm2.
- The coefficient of variation of measurements does not exceed 5% in the concentration range of 20–200 pg/mm2 and 10% in the range of 2–20 pg/mm2.
- LICHT has been developed to detect cholera toxin, staphylococcal enterotoxin type B, plague pathogen cells (vaccine variant EV), anthrax pathogen spores, as well as antigens of the Crimean-Congo hemorrhagic fever pathogen virus. Further studies should address the extension of the LICHT range for the detection of pathogens.
- The sensitivity of LICHT based on microspheres containing europium complexes during instrument registration is 20–128 times higher than that of immunochromatographic tests based on AuNPs, designed on the basis of the same specific immunoglobulins and analytical membranes.
References
1. Hou FP, Bai MY, Zhang Y, Liu HY, Sun SQ, Guo HC. Fluorescent immunochromatographic assay for quantitative detection of the foot-and-mouth disease virus serotype O antibody. Microchem. J. 2020;155:104690. https://doi.org/10.1016/j.microc.2020.104690
2. Kim TK, Oh S, Hong SC, Mok YJ, Choi EY. Point-of-Care Fluorescence Immunoassay for Cardiac Panel Biomarkers. J. Clin. Lab. Anal. 2014;28:419–27. https://doi.org/10.1002/jcla.21704
3. Gao YM, Wei JC, Mak PU, Vai MI, Du M, Pun SH. Development of a Calibration Strip for Immunochromatographic Assay Detection Systems. Sensors. 2016;16:1007. https://doi.org/10.3390/s16071007
4. Raeisossadati MJ, Danesh NM, Borna F, Gholamzad M, Ramezani M, Abnous K, Taghdisi SM. Lateral flow-based immunobiosensors for detection of food contaminants. Biosens.Bioelectron. 2016;86:235–46. https://doi.org/10.1016/j.bios.2016.06.061
5. Wu Y, Sun J, Huang X, Lai W, & Xiong Y. Ensuring food safety using fluorescent nanoparticles-based immunochromatographic test strips. Trends in Food Science & Technology. 2021; 118:658–78. https://doi.org/10.1016/j.tifs.2021.10.025
6. Sanchis A, Salvador JP, Marco MP. Multiplexed immunochemical techniques for the detection of pollutants in aquatic environments. TrAC Trends Anal. Chem. 2018;106:1–10. https://doi.org/10.1016/j.trac.2018.06.015
7. Berlina AN, Taranova NA, Zherdev AV, Vengerov YY, Dzantiev BB. Quantum dotbased lateral flow immunoassay for detection of chloramphenicol in milk. Analytical and Bioanalytical Chemistry. 2013;405(14):4997–5000. https://doi.org/10.1007/s00216-013-6876-3
8. Taranova NA, Berlina AN, Zherdev AV, Dzantiev BB. “Traffic light” immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosensors and Bioelectronics. 2015;63:255–61. https://doi.org/10.1016/j.bios.2014.07.049
9. Yang J.C., Wang K., Xu H., Yan W.Q., Jin Q.H., Cui D.X. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review. Talanta. 2019;202:96–110. https://doi.org/10.1016/j.talanta.2019.04.054
10. Bajusheva VV, Belozerova OA, Cherednichenko AG. Synthesis and luminescent properties of rare earth metal complexes (REM) with substituted 1,10-phenanthroline and β-diketones. Uspekhi v Khimii i Khimicheskoi Tekhnologii Magazine. 2014;28(6):16–8 (In Russ.). EDN: STFWMN
11. Yarkov SP, Tretyakov SI, Shilenko IV, Ishkov YuN, Styazhkin KK. Identification of staphylococcal enterotoxin B in dairy products by immunochromatography with visual and digital video detection. Extreme Medicine. 2023;3:86–91 (In Russ.). https://doi.org/10.47183/mes.2023.039
12. Jiao X, Peng T, Liang Z, Hu Y, Meng B, Zhao Y, et al. Lateral Flow Immunoassay Based on Time-Resolved Fluorescence Microspheres for Rapid and Quantitative Screening CA199 in Human Serum. Int. J. Mol. Sci. 2022;23:9991–9. https://doi.org/10.3390/ijms23179991
13. Eremkin AV, Ipatov SS, Kuklina GV, Pechenkin DV, Kytmanov AA, Tikhvinskaya OV, et al. Development of ELISA test-systems and immune-chromatographic reagent panel designed for the detection of staphylococcal enterotoxins of A and B types. Problems of Particularly Dangerous Infections. 2021;2:94–9 (In Russ.). https://doi.org/10.21055/0370-1069-2021-2-94-99
14. Yarkov SP, Tretyakov SI, Shaulina EK, Brovkina AN, Khramov EN. Elevation the sensitivity of immunochromatographic tests to identify the causative agent of anthrax and staphylococcal enterotoxin type B based on silver amplification and instrumental recording. Extreme medicine. 2019;21(3):122–31 (In Russ.). EDN: KSFFST
15. Lapaev DV, Ziyatdinova RM, Knyazev AA, Galyametdinov YuG, Nikiforov VG, Lobkov VS. Mechanism of temperature quenching of luminescence in a glassy film of the β-diketonate complex of europium (III). Bulletin of the Technological University. 2018;21(4):13–8 (In Russ.). EDN: XPSTYL
16. Xie QY, Wu YH, Xiong QR, Xu HY, Xiong YH, Liu K, et al. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014;54:262–5. https://doi.org/10.1016/j.bios.2013.11.002
17. Gupta, R., Gupta, P., Wang, S. et al. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 2023;7:1556–70. https://doi.org/10.1038/s41551-022-01001-1
About the Authors
S. P. YarkovRussian Federation
Sergey P. Yarkov
Moscow
S. I. Tretyakov
Russian Federation
Sergey I. Tretyakov
Moscow
I. V. Shilenko
Russian Federation
Inessa V. Shilenko
Moscow
E. K. Shaulina
Russian Federation
Ekaterina K. Shaulina
Moscow
A. Mandaji
Russian Federation
Angelina Mandaji
Moscow
D. A. Zenkov
Russian Federation
Denis A. Zenkov
Moscow
Yu. N. Ishkov
Russian Federation
Yury N. Ishkov
Moscow
K. K. Styazhkin
Russian Federation
Konstantin K. Styazhkin
Moscow
Supplementary files
Review
For citations:
Yarkov S.P., Tretyakov S.I., Shilenko I.V., Shaulina E.K., Mandaji A., Zenkov D.A., Ishkov Yu.N., Styazhkin K.K. Luminescent immunochromatography based on Eu³⁺ coordination compounds for detection of pathogenic microorganisms and bacterial toxins. Extreme Medicine. 2025;27(1):107-114. https://doi.org/10.47183/mes.2025-257
















