Scroll to:
Applicability of individual metabolites of the tricarboxylic acid cycle in athletes (A literature review)
https://doi.org/10.47183/mes.2025-288
Abstract
Introduction. Various metabolites of the tricarboxylic acid cycle (Krebs cycle, TCA cycle, TCAC) find application in medicine. The emergence of improved chemical synthesis methods and the more affordable production of individual TCA metabolites make them promising candidates for developing effective compositions capable of increasing the adaptive potential of the human body.
Objective. Identification of the physiological effects of the main TCA metabolites. This knowledge is important for informed application of TCA metabolite-based products in the medical and biological support of athletes.
Discussion. The conducted literature review investigated the physiological effects of TCA metabolites — energy metabolism substrates — used in sports medicine. At present, succinate, citrate, malate, and oxaloacetate have found reasonable use. A number of publications have reported the anti-catabolic effect of alpha-ketoglutarate; however, the current level of evidence is insufficient. Isocitrate dehydrogenase is promising for use in sports medicine, which substantiates its further detailed study.
Conclusions. Due to their physiological effects, the majority of TCA metabolites can be used in the compositions of antihypoxic, antioxidant, neuroprotector, and metabolic correction agents. A number of TCA metabolites are promising substances for creating new products for the medical and biological support of athletes, which validates additional research of their physiological effects.
Keywords
For citations:
Yashin T.A., Grishina Zh.V., Parastaev S.A., Zholinsky A.V. Applicability of individual metabolites of the tricarboxylic acid cycle in athletes (A literature review). Extreme Medicine. 2025;27(2):249-256. https://doi.org/10.47183/mes.2025-288
INTRODUCTION
In modern sports medicine, the search for new safe substances capable of optimizing metabolic processes in athletes experiencing high professional loads represents a relevant research direction. The incidence of energy metabolism disorders with profound hypoxic and ischemic changes can be reduced through the timely use of antihypoxic agents that directly affect redox processes [1–3].
The metabolites of the tricarboxylic acid cycle (TCA cycle, Krebs cycle, citric acid cycle, TCAC) are promising agents for preventing energy metabolism disorders resulting from professional sports loads.
The tricarboxylic acid cycle is the central pathway for the conversion of organic acids during the anaerobic oxidation of glucose in the cell with the release of energy in the form of ATP. This cycle plays a key role in cellular respiration, being the main source of energy in aerobic conditions1. Under anaerobic conditions, glucose is oxidized to pyruvic acid (Pyr), which, under the action of enzymes, is converted into acetyl-coenzyme A (acetyl-CoA), where the TCA begins (Fig. 1).
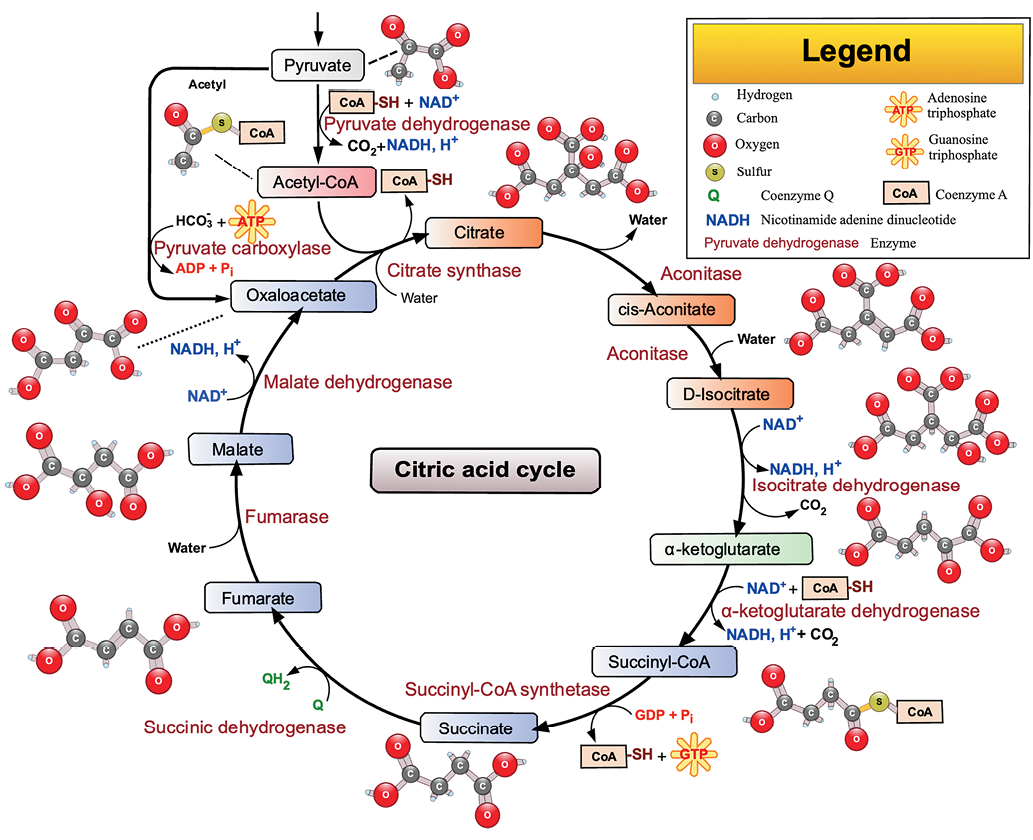
Figure prepared by the authors using data from Refs.2
Fig. 1. Tricarboxylic acid cycle scheme
The TCA cycle in athletes has a number of specific characteristics. Thus, during physical exercise with an intensity above 50% of maximal oxygen uptake (VO2 max), the total concentration of TCA intermediates in skeletal muscles increases. This phenomenon is referred to as anaplerosis [4–6]. Anaplerosis is commonly associated with an increase in the activity of the enzyme alanine aminotransferase (ALT) due to increased availability of pyruvate. As a result of glycolysis, its formation rate exceeds the oxidation rate: glutamate + pyruvate ↔ alanine + alpha-ketoglutarate. One currently leading hypothesis explaining the phenomenon of anaplerosis under the action of high athletic loads consists in the following. The observed increase in the pool of TCA intermediates in muscles is necessary to achieve higher rates of providing the aerobic pathway of energy formation. That is why an increase in the pool of TCA intermediates by exogenous addition of individual TCA metabolites may be a factor in improving the peak muscle oxidative capacity, which is especially important for professional athletes [5][6].
In this study, we aim to identify the physiological effects the main TCA metabolites. This knowledge forms the basis for developing TCA metabolite-based products for the medical and biological support of athletes.
MATERIALS AND METHODS
The relevant scientific publications were retrieved from electronic bibliographic databases in the Russian (eLibrary, CyberLeninka) and English (Web of Science, Scopus, PubMed) languages. The search queries included the following keywords: Krebs cycle; tricarboxylic acids; tricarboxylic acid cycle; energy metabolism; energy metabolism substrates; high-performance sports; antioxidants; antihypoxants; isocitric acid. The search depth was 30 years. The inclusion criteria were the availability of data on the results of randomized controlled trials, including data from preclinical studies.
RESULTS AND DISCUSSION
The tricarboxylic acid cycle is the central link in the cell energy metabolism. A number of substances related to the TCA metabolism have found application in medicine in general and in sports medicine in particular. Such substances can be used by athletes to increase the adaptive potential of the body, optimize energy production in muscle cells, and protect the body from the effects of various adverse factors, including increased stress. Further, we summarize the information on TCA metabolites.
Citric acid (citrate) is the first TCA metabolite formed by condensation of two Pyr molecules3. An increase in the amount of glucose leads to the formation of a large amount citrate. As a result, the activity of phosphofructokinase is inhibited and glycolysis slows down, which is energetically beneficial for the cell. The high concentration of citrate indicates the presence of a large supply of precursor molecules; therefore, phosphofructokinase does not release fructose-6-phosphate molecules into glycolysis, thus saving energy substrates [7].
Citric acid and citrate preparations are widely used in medicine. For example, citrates are used to alkalize urine (as an alternative to sodium bicarbonate) under the conditions where, for health reasons, it is desirable to maintain its pH at an alkaline level for a certain period of time. This property is relevant for athletes who, when taking citric acid derivative-based medications, experience an increase in the buffer capacity of body fluids and, therefore, a delayed onset of fatigue due to a decrease in the body acidity level [8][9].
Citrate also exhibits antioxidant properties, having a synergistic effect with vitamin E [8][9]. The use of citrate reduces the load on the body’s antioxidant system (AOS), which is manifested by a decrease in the activity of superoxide dismutase (SOD), catalase, glutathione peroxidase, a reduced glutathione level, as well as the activity of some NADPH-generating enzymes, including during strenuous physical work [8]. In addition, citrate is capable of exhibiting cytoprotective properties and act as an activator of fatty acid biosynthesis and a supplier of acetyl fragments for cell membrane repair [7]. Another important citrate property is its ability to retain magnesium inside mitochondria, thereby protecting them from damage [9][10]. All of the above-mentioned physiological properties of citrate and its derivatives make the use of medications on its basis highly promising for mitigating the effects of high loads typical of professional sports.
Isocitrate (isocitric acid, ICA) is the next important TCA metabolite. ICA is synthesized from citric acid through the intermediate cis-aconitic acid under the action of the aconitase enzyme. It is believed that the reaction of conversion of isocitric acid to alpha-ketoglutarate is the reaction that limits the rate of the entire TCA cycle. Despite this fact, isocitric acid is currently one of the least studied TCA metabolites in terms of its effects on the human body. For a long time, isocitrate was used only as a specific biochemical reagent for analyzing the activity of aconitate hydratase, NAD-isocitrate dehydrogenase, NADP-isocitrate dehydrogenase, isocitrate lyase, and other enzymes [11][12]. Relatively recently, ICA has been studied as a natural preventive and therapeutic agent, with its effectiveness in the treatment of iron deficiency anemia and therapeutic thrombolysis being reported [12].
A number of studies have demonstrated the effectiveness of isocitric acid in anemia of chronic disease and inflammation (ACDI), as well as in the setting of professional sports activities [13, 14]. Using isocitrate, erythropoiesis can be therapeutically manipulated without using iron preparations. This is especially true in cases where the body’s iron load is undesirable or ineffective [13][14].
In an experimental rat model, the antioxidant properties of isocitrate were demonstrated. Monopotassium isocitrate was found to be a more effective antioxidant than ascorbic acid. Monopotassium isocitrate also mitigated the neurotoxic effect of lead and molybdenum salts, reduced learning and memory inhibition in rats poisoned with heavy metals, and counteracted oxidative stress caused by heavy metals [15].
In the study [16], a 10-day intake of isocitric acid during antihypertensive therapy showed a stress-protective effect. This is likely to be related to the capacity of isocitrate to influence the processes of excitation and inhibition in the central nervous system. The researchers also noted a previously undescribed antihypertensive effect, which was manifested in a significant decrease in the average daily diastolic blood pressure when taking isocitric acid.
In addition, the isocitrate dehydrogenase (IDH) enzyme plays a central role in the TCA-cycle control during physical exercise [17]. IDH isoforms play an important role in protecting cells from oxidative damage due to the reaction of direct oxidative decarboxylation. In addition, these may serve as a source of NADPH [17].
Another area of research into the physiological effects of isocitrate concerns its antihypoxic effects. Hypoxia affects the enzymes involved in the TCA cycle in different ways. In particular, under hypoxia conditions, aconitase is suppressed, while IDH is not affected or activated. IDH was shown to be essential for alternative metabolic pathways that support cell function under hypoxic conditions. It is assumed that the addition of exogenous isocitrate removes aconitase inhibition and serves as an IDH substrate to provide an alternative energy source in hypoxia [18][19]. This isocitrate effect can be used in sports where hypoxic conditions are present, as well as during training in the mountains.
Isocitrate is a promising substance for the creation of medications and specialized food products for use in the medical and biological support of athletes. However, for the justified use of this organic acid in sports medicine, further study of its effect on the body is required, including under the action of increased physical and psychoemotional stress.
Alpha-ketoglutaric acid (alpha-ketoglutarate, AKG, 2-oxoglutarate) is an important intermediate in the TCA cycle, passing from isocitrate to succinyl-CoA [15][19]. Anaplerotic reactions can replenish the TCA cycle at this stage by synthesizing alpha-ketoglutarate as a result of glutamate transamination or under the action of glutamate dehydrogenase. Another function of this metabolite is to prevent nitrogen overload of cells through the capacity of alpha-ketoglutorate to combine with excess nitrogen and directs it into the urea cycle [19]. In addition, alpha-ketoglutarate reacts with glutamine to form the excitatory neurotransmitter glutamate. Then glutamate can be decarboxylated (vitamin B6 is required) into gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter [20].
In the work [21], alpha-ketoglutaric acid was shown to exhibit antioxidant properties in mice, being capable of preventing damage to mitochondrial DNA caused by free radicals in nerve cells.
Alpha-ketoglutaric acid can also function as a signaling molecule by regulating the G protein function. Signaling through this pathway mobilizes intracellular Ca2+, which acts as a diffusive second messenger regulating a wide range of vital cell functions, including cellular metabolism and growth, as well as cell division and differentiation [22]. There are also studies demonstrating the effectiveness of AKG in accelerating tissue repair after surgery, injury, and burns [19][23].
As a precursor to glutamine, alpha-ketoglutarate is a molecule with a high potential for correcting conditions with increased protein catabolism, including those caused by prolonged aerobic exercise in athletes. AKG supplements were shown to improve the body nitrogen balance and contribute to maintaining the level of anabolic hormones and hormone-like compounds (insulin, growth hormone, and insulin-like growth factor) during surgical interventions, injuries, and burns [23]. In addition, AKG may protect liver cells from damage and prevent a decrease in the activity of the cytochrome P-450 family. This hepatoprotective effect may also be relevant in sports medicine [23].
The positive effect of alpha-ketoglutaric acid on bone metabolism described in a number of studies suggests its potential use in the prevention of bone matrix formation disorders, in the treatment of diseases with progressive bone loss, such as osteoporosis, or in improving the body’s bone mass, which is also relevant for professional athletes [24].
The study [25] showed that a significant accumulation of blood AKG is a metabolic signal of the effectiveness of resistance training. Interestingly, its plasma level negatively correlates with the body mass index. In vivo experiments in mice found that an increase in circulating AKG in the blood caused by its exogenous intake into the body promotes hypertrophic changes in muscle tissue, thermogenesis, due to brown fat and lipolysis of white fat. AKG was also found to stimulate the release of adrenaline. The results of this study demonstrate an underestimated mechanism of AKG as a molecule in adrenal stimulation for muscle hypertrophy and fat loss in resistance training [25].
Currently, in the practice of sports medicine, AKG is used in combination with the L-arginine amino acid as a component of specialized food products under the common name of AAKG. In some studies, the anabolizing and anti-catabolic effects of AKG application were identified [26]. However, currently, due to insufficient knowledge and a limited number of studies on athletes, AAKG belongs to specialized food products with a low level of evidence-based effectiveness when used in professional sports [27].
Succinic acid (SA, succinate) is formed from alpha-ketoglutarate via succinyl-CoA. All metabolic pathways that are interconnected with the TCA cycle, including the metabolism of carbohydrates, amino acids, fatty acids, cholesterol, and heme, depend on the temporary formation of succinate.
Succinate can exit the mitochondrial matrix and function both in the cytoplasm and in the extracellular space, altering gene expression patterns, modulating the epigenetic landscape, or demonstrating hormone-like signaling. For example, in adipocytes, the signaling cascade activated by succinate inhibits lipolysis. Most often, succinate signaling occurs in response to hypoxic conditions [26].
When oxidized, succinate monopolizes the respiratory chain, which leads to rapid ATP resynthesis by cells and increases the amount of reduced mitochondrial NAD+ more markedly than other TCAC substrates, stimulating the course of reductive synthesis in the cell and supporting calcium transport. Its positive effect on organ functions is associated with an energizing effect on the functional state of structures that exert a central regulatory effect [28].
This metabolite is the re-entry point for the GABA shunt into the TCA cycle, where GABA is synthesized and processed, which explains its antistress effect [29]. In addition, succinate can enhance adaptive immunity by triggering the activity of antigens that activate T cells. It was also shown that accumulated succinate regulates the production of inflammatory cytokines [30].
The researchers in [30] studied the antihypoxic and antioxidant effects of succinate. These effects are not based only on its ability to activate IDH (the ATP resynthesis pathway), reduce the level of NAD-dependent TCA substrates and fatty acids, but are also associated with the stimulation of cytochrome oxidase, which is a key enzyme of the mitochondrial respiratory chain. Succinate is helpful in normalizing the concentration of histamine and serotonin in the blood and epidermis, while having a beneficial effect on the microcirculatory system without affecting blood pressure (BP) and heart function.
The work [31] addressed the hepatoprotective effect of succinate, which consists in activating the enzyme succinate dehydrogenase (SDH) in the mitochondria of hepatocytes. This leads to normalization of urea synthesis disorders, mitigation of hepatic cholestasis, prevention of fatty degeneration of the liver, and the development of collagenous tissue in the liver.
The adaptogenic effect of succinate was described in experiments using models of immobilization stress and stress provoked by burns, electric shock, and hypothermia [31]. It is also known that succinate promotes accelerated recovery during heavy physical exertion.4
One of the factors that limit the use of succinate in sports medicine is its bioavailability, which is lower than that of fumarate, malate, or citrate. To increase the bioavailability of succinate, its compounds with other TCA metabolites in the form of salts can be used.5
Currently, succinate is widely applied in domestic sports medicine. Thus, Mexidol® (3-hydroxy-6-methyl-2-ethylpyridine succinate), a medication of the metabolic type of action has a powerful inhibitory effect on lipid peroxidation processes, as well as the effects of neutralizing free radicals and activating SOD. Mexidol® promotes the activation of the succinate oxidase oxidation pathway, due to which a certain level of oxidative phosphorylation is maintained in mitochondria at the initial stages of hypoxia under conditions of inhibition of NAD-dependent oxidation [32]. However, it should be noted that the use of Mexidol® by professional athletes is allowed only in tablet form, whereas intravenous infusions and/or injections of more than 100 mL over a 12h period are prohibited by the World Anti-Doping Agency (WADA) Code. The same rule applies to the Reamberin® medication, which contains meglumine sodium succinate in its composition.6
In addition to Mexidol®, Cytoflavin®, the active ingredients of which include succinic acid in combination with inosine, nicotinamide, and riboflavin, is currently widely used as a component of the medical and biological support for athletes in Russia. Cytoflavin® was found to enhance cellular respiration during strenuous physical activity, providing an optimal level of oxygen uptake by cells [32][33]. The course use of Cytoflavin® was found to have a positive effect on metabolic processes in the body, such as supporting protein-synthetic function, promoting the absorption of glucose and fatty acids by cells, improving cellular energy supply, and restoring the activity of enzymes of the antioxidant system. This medication can be classified as a drug exhibiting adaptogenic and stress-protective properties [32][34][35]. The study [33] revealed the positive effects of Cytoflavin® on the performance of professional hockey players in the pre-competition period [33][34].
Fumaric acid (fumarate) is formed as a result of the oxidation of succinate with the participation of the SDH enzyme, which is also involved in the mitochondrial electron transport chain (respiratory complex II). Fumarate functions as an intermediate product of urea synthesis and oxidation of phenylalanine, tyrosine, leucine, tryptophan, and lysine [36][37]. There is evidence that fumarate derivatives act as appetite-enhancing agents and possess antifungal effects, being also used as tranquilizers and radiopaque medications, as well as agents for blood-clotting disorders (bencyclane hydrofumarate) and rhinitis [36–38].
Fumarate penetrates readily through membranes and is easily disposed of in mitochondria. This compound, similar to lactate and sodium acetate, helps eliminate acidemia by chemically neutralizing acidic metabolic products. However, fumarate has an advantage over lactate and acetate, since it is metabolized during severe hypoxia. Moreover, its utilization is accompanied by the formation of ATP [37, 38].
Fumarate-based medications that support the activity of the succinate link during hypoxia of various origins is increasingly finding practical application as antihypoxic agents. One of these medications is mafusol (1 L of an aqueous solution for injection contains 6.0 g sodium chloride, 0.3 g potassium chloride, 0.12 g magnesium chloride and 14.0 g sodium fumarate) [40]. Mafusol can also be used in sports medicine provided that it does not contradict the following WADA anti-doping rule: intravenous infusions and/or injections in a volume of more than 100 mL during a 12-hour period are prohibited.
Malic acid (malate) is formed from fumarate under aerobic conditions. Malate has the properties of a cellular protector, being also capable of increasing the activity of enzyme complexes, such as SOD and glutathione peroxidase, by enhancing the expression of messenger RNA [3].
It was noted that the activity of the malate-aspartate transporter in the heart muscle is more than 10 times higher than that of all other known electron transport systems. The study of cardiomyocytes in ischemia and at the time of post-ischemic reperfusion showed the tremendous importance of this system in the adequate supply of cells with energy. In addition, the malate-aspartate mechanism is a link in antioxidant protection and an agent in insulin synthesis [3][39][40].
Malate performs various functions of switching metabolic pathways: it participates in glycolysis, beta-oxidation of fatty acids, synthesis of amino acids, playing an important role in transport communication between mitochondria and the cytosol, exerting anaplerotic or cataplerotic effects on the central nervous system [3][39][40]. It is known that lettuce can indirectly have an antihypoxic effect by pre-dehydrating to fumarate [3][39][40].
In sports medicine, a compound of malic acid with the citrulline amino acid is used. This compound has a tonic effect, reducing fatigue and increasing endurance [41][42]. An example of such a medication is Stimol®, which is widely used by sports physicians to accelerate the recovery of an athlete after heavy loads. In [43], this medication accelerated lactate excretion from muscles in sprinting athletes.
Oxaloacetic acid (OA, oxaloacetate) is another important metabolite of the TCA cycle. Its synthesis is maintained mainly due to pyruvate carboxylation; therefore, a reduced intensity of glycolysis during hypoglycemia and depletion of glycogen stores leads to a deficiency of pyruvate and, as a result, to a lack of oxaloacetate. This limits not only the entry of acetyl-CoA into the TCA cycle, but also the course of other important adaptive reactions [3][44].
Oxaloacetate participates in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, and fatty acid synthesis. It is important for the formation of essential and non-essential amino acids, including aspartate, asparagine, methionine, lysine, and threonine [44][45].
Bioenergetic medications based on oxaloacetate derivatives were developed to increase the cell energy level. Such medications exhibit protective and promitochondrial effects, preventing neuroinflammation and non-degeneration [44, 45]. Oxaloacetate acts as a neuroprotector due to its ability to reduce the content of glutamate in the brain as a result of activation of the glutamate oxaloacetate transaminase enzyme, which catalyzes the reversible conversion of oxaloacetate and glutamate into aspartate and alpha-ketoglutarate. It promotes recovery after traumatic brain injury (TBI), causing neurorehabilitation effects, which also has prospects for use in sports medicine in rehabilitation after TBI sustained during training or competition [45].
Among other properties of oxaloacetate, its participation in the processes of gluconeogenesis and glycerogenesis should be noted. Oxaloacetate is capable of increasing the volume of mitochondria in striated muscles, which has a positive effect on endurance by reducing muscle fatigue [44][45]. Another important advantage associated with the introduction of oxaloacetate is an increase in mitochondrial biogenesis [45].
CONCLUSION
The majority of TCA metabolites are actively used in medicine for antihypoxic, antioxidant, neuroprotector, and metabolic therapies. Succinate and its derivatives (Cytoflavin®, Mexidol® ergogenic, antihypoxic medications), citrate (as a buffer agent and magnesium carrier), alpha-ketoglutarate (to suppress catabolism, stimulate anabolism, hepatoprotection), malate (in the form of citrulline malate to increase endurance, the Stimol® medication) have found their use in sports medicine, oxaloacetate (as a component of medications whose action is aimed at recovery after TBI, reducing fatigue, and metabolic regulation of gluconeogenesis).
At present, there is a lack of data on a number of TCA metabolites, which limits their application in the medical and biological support of professional athletes. For example, alpha-ketogluturate as a component of specialized food products in combination with the L-arginine amino acid, despite the anti-catabolic and anabolic effects shown in some studies, currently has a low degree of evidence-based effectiveness when used in professional sports. Isocitrate, despite its safety and promise as a component of medications and specialized food products used in sports medicine, has not yet been widely used due to its rather expensive synthesis. However, the recent progress in making the production of isocitrate more affordable offers the opportunity for its wider use in the practice of sports medicine physicians.
1. Nesen EN, Volkov NI, Osipenko AA, Korsun SN. Biochemistry of muscle activity. Kyiv: Olympic literature; 2013.
2. https://chemicalportal.ru/compounds/tsikl-trikarbonovyh-kislot
3. Kulinenkov OS, Lapshin IA. Biochemistry in the practice of sports.: Guide. Moscow.: Sport; 2019.
4. Isakov VA, Sologub TV, Kovalenko AL, Romantsov MG. Reamberin in the treatment of critical conditions. Guide for doctors. St. Petersburg.: JV Minimax; 2001.
5. Ibid.
6. Ibid.
References
1. Gibala MJ, Peirce N, Constantin-Teodosiu D, Greenhaff PL. Exercise with low muscle glycogen augments TCA cycle anaplerosis but impairs oxidative energy provision in humans. J Physiol. 2002;540(3):1079–86. https://doi.org/10.1113/jphysiol.2001.012983
2. Mayevsky EI, Grishina EV. Biochemical basis of the mechanism of action of fumarate-containing drugs. Medline.ru. Russian biomedical journal. 2017;18:50–80 (In Russ.). EDN: YRIDMT
3. Kolotyeva NA, Gilmiyarova FN. The role of small molecules in the regulation of metabolism (literature review). Clinical laboratory diagnostics. 2019;64(12):716–22 (In Russ.).
4. Mourtzakis M, Graham TE, González-Alonso J, Saltin B. Glutamate availability is important in intramuscular amino acid metabolism and TCA cycle intermediates but does not affect peak oxidative metabolism. Journal of Applied Physiology. 2008;105(2):547–54. https://doi.org/10.1152/japplphysiol.90394.2008
5. Gibala MJ, González-Alonso J, Saltin B. Dissociation between muscle tricarboxylic acid cycle pool size and aerobic energy provision during prolonged exercise in humans. J Physiol. 2002;545: 705–13. https://doi.org/10.1113/jphysiol.2002.028084
6. Howarth KR, LeBlanc PJ, Heigenhauser GJF, Gibala MJ. Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J Appl Physiol. 2004;97:579–84. https://doi.org/10.1152/japplphysiol.01344.2003
7. Hu YY, Rawal A, Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proceedings of the National Academy of Sciences. 2010;107(52):22425–9. https://doi.org/10.1073/pnas.1009219107
8. Safonova OA, Popova TN, Saidi L. Activity of some NADPH-producing enzymes in the tissues of rats during toxic hepatitis and the action of citrate. Basic research. 2008;7:39–40 (In Russ.). EDN: JKFPRN
9. Gromova OA, Torshin IYu. Grishina TR. World experience in the use of magnesium citrate in medicine. Difficult patient. 2010;8:35–44 (In Russ.). EDN: OGBOIV
10. Westergaard N, Waagepetersen HS, Belhage B, Schousboe A. Citrate a Ubiquitous Key Metabolite with Regulatory Function in the CNS. Neurochem Res. 2017;42(6):1583–8. https://doi.org/10.1007/s11064-016-2159-7
11. Finogenova TV, Morgunov IG, Kamzolova SV, Chernyavskaya OG. Organic acid production by the yeast Yarrowia lipolytica: a review of prospects. Appl Biochem Microbiol. 2005;41:418–25. https://doi.org/10.1007/s10438-005-0076-7
12. Yang J, Kim MJ, Yoon W, Kim EY, Kim H, Lee Y, Min B, Kang KS, Son JH, Park HT, Chung J, Koh H. Isocitrate protects DJ-1 null dopaminergic cells from oxidative stress through NADP+-dependent isocitrate dehydrogenase (IDH). PLoS Genet. 2017;13(8):e1006975. https://doi.org/10.1371/journal.pgen.1006975
13. Richardson CL, Delehanty LL, Bullock GC, Rival CM, Tung KS, Kimpel DL, Gardenghi S, Rivella S, Goldfarb AN. Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J Clin Invest. 2013;123(8):3614–23. https://doi.org/10.1172/JCI68487
14. Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–61. https://doi.org/10.1182/blood-2010-05-286260
15. Morgunov IG, Karpukhina OV, Kamzolova SV, Samoilenko VA, Inozemtsev AN. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep Biochem Biotechnol. 2017;48(1):1–5. https://doi.org/10.1080/10826068.2017.1381622
16. Pribilova SA, Peskov AB, Khokhlov MP, Sytnik VV, Lykova NS, Kerova IR. Results of the use of isocitric and αketoglutaric acids in the complex therapy of arterial hypertension. Bulletin of new medical technologies. 2012;1:31–35 (In Russ.). EDN: PKJWGR
17. Howlett RA, Willis WT. Fiber-type-related differences in the enzymes of a proposed substrate cycle. Biochim Biophys Acta. 1998;25;1363(3):224–30. https://doi.org/10.1016/s0005-2728(98)00002-4
18. Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–4. https://doi.org/10.1038/nature10602
19. Zdzisińska B, Żurek A, Kandefer-Szerszeń M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch Immunol Ther Exp (Warsz). 2017;65(1):21–36. https://doi.org/10.1007/s00005-016-0406-x
20. Newsholme P, Procopio J, Ramos Lima MM, et al. Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem. 2009;21:1–9. https://doi.org/10.1002/cbf.1003
21. Yamamoto H, Mohanan PV. Effect of α-ketoglutarate and oxaloacetate on brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Toxicol Lett. 2003;143:115–22. https://doi.org/10.1016/S0378-4274(03)00114-0
22. He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–93. https://doi.org/10.1038/nature02488
23. Cynober L, Lasnier E, Le Boucher J, et al. Effect of ornithine alpha-ketoglutarate on glutamine pools in burn injury: evidence of component interaction. Intensive Care Med. 2007;33:538–41. https://doi.org/10.1007/s00134-006-0511-0
24. Tatara MR, Krupski W, Tymczyna B, et al. Effects of combined maternal administration with alpha-ketoglutarate (AKG) and beta-hydroxy-methylbutyrate (HMB) on prenatal programming of skeletal properties in the offspring. Nutr Metab. 2012;9:39. https://doi.org/10.1186/1743-7075-9-39
25. Yuan Y, Xu P, Jiang Q, Cai X, Wang T, Peng W. Exercise-induced α-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO J. 2020;39(7):e103304. https://doi.org/10.15252/embj.2019103304
26. Bill Campbell MS. et al. Pharmacokinetics, safety, and effects on exercise performance of l-arginine α-ketoglutarate in trained adult men. Nutrition. 2006;22(9):872–81. https://doi.org/10.1016/j.nut.2006.06.003
27. Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2008;15:38. https://doi.org/10.1186/s12970-018-0242-y
28. Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochimica et Biophysica Acta (BBA) Bioenergetics. 1857(8):1086–101. https://doi.org/10.1016/j.bbabio.2016.03.012
29. Olsen RW, DeLorey TM, In S, Agranoff BW, Albers RW, et al. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th ed. Philadelphia: Lippincott-Raven;1999.
30. Ariza AC, Deen PM, Robben JH. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Frontiers in Endocrinology. 2012;3:22. https://doi.org/10.3389/fendo.2012.00022
31. de Castro FМ, Aguiar CJ, da Rocha Franco JA, Gingold RN, Leite MF. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Communication and Signaling. 2016;14:3. https://doi.org/10.1186/s12964-016-0126-1
32. Galenko-Yaroshevsky PA, Chekman IS, Gorchakova NA. Essays on the pharmacology of metabolic therapy. Moscow: Medicine; 2001 (In Russ.). EDN: QIYLHN
33. Kurshev VV, Zaborova VA, Achkasov EE, Nebozhaeva SF. Dynamics of laboratory parameters under the influence of metabolic correction in highly qualified hockey players in the preparatory period. Current issues of diagnosis and treatment: Collection of scientific papers. Moscow: Medicine; 2018 (In Russ.). EDN: XWUSPZ
34. Achkasov EE, Kurshev VV, Zaborova VA, Nebozhaeva SF. The influence of stepwise cytoflavin therapy on the dynamics of laboratory parameters in professional athletes — hockey players at the first stage of preparation for the playing season. Clinical medicine. 2018;96(4):354–60 (In Russ.). https://doi.org/10.30906/0869-2092-2017-80-10-36-39
35. Kosinets VA, Stolbitsky VV, Shturich IP. Experience of using cytoflavin in sports nutrition. Clinical medicine. 2012:90(7):56–9 (In Russ.). EDN: RBINKP
36. Sukhomlin AK, Velikiy KF, Alekseeva NN, Alekseeva NN. Efficacy of fumarate-containing antihypoxants in the correction of systemic hypoxia and endogenous intoxication in general peritonitis (experimental and clinical studies). Journal of International Medicine. 2016;2:67–70 (In Russ.). EDN: YJQLXB
37. Shakhmardanova SA, Gulevskaya ON, Khananashvili YA, Zelenskaya AV, Nefedov DA, Galenko-Yaroshevsky PA. Preparations of succinic and fumaric acids as a means of prevention and treatment of various diseases. Journal of Basic Medicine and Biology. 2016;3:16–30 (In Russ.). EDN: XQSIDV
38. Slepneva LV, Khmylova GA, Alekseeva NN, Selivanova EA. Antihypoxic drug. Patent of the Russian Federation No. 2189813;2002 (In Russ.).
39. Safonova OA, Popova TN, Matasova LV. Some kinetic parameters and regulatory properties of NADP-dependent malate dehydrogenase from rat cardiomyocytes under normal conditions and ischemia. VSU Bulletin. Chemistry. Biology. Pharmacy. 2004;1:142–6 (In Russ.). EDN: OITHUB
40. Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Sci Transl. Med. 2016;8:326. https://doi.org/10.1126/scitranslmed.aac7410
41. Bendahan D, Mattei JP, Ghattas B. Citrulline/malate promotes aerobic energy production in human exercising muscle. British Journal of Sports Medicine. 2002;36:282–9. https://doi.org/10.1136/bjsm.36.4.282
42. Pérez-guisado J, Jakeman PM. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J Strength Cond Res. 2010;24(5):1215–22. https://doi.org/10.1519/JSC.0b013e3181cb28e0
43. Divito B, McLaughlin M, Jacobs I. The Effects of L-Citrulline on Blood-Lactate Removal Kinetics Following Maximal-Effort Exercise. J Diet Suppl. 2022;19(6):704–16. https://doi.org/10.1080/19390211.2021.1926392
44. Zlotnik A, Gruenbaum SE, Artru AA, Rozet I, Dubilet M. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: evidence from the use of maleate. J Neurosurg Anesthesiol. 2009;21(3):235–41. https://doi.org/10.1097/ANA.0b013e3181a2bf0b
45. Wilkins HM, Koppel S, Carl SM, et al. Oxaloacetate enhances neuronal cell bioenergetic fluxes and infrastructure. J Neurochem. 2016;137(1):76–87. https://doi.org/10.1111/jnc.13545
About the Authors
T. A. YashinRussian Federation
Timofey A. Yashin
Moscow
Zh. V. Grishina
Russian Federation
Zhanna V. Grishina, Cand. Sci. (Biol.)
Moscow
S. A. Parastaev
Russian Federation
Sergey A. Parastaev, Dr. Sci. (Med.), Professor
Moscow
A. V. Zholinsky
Russian Federation
Andrey V. Zholinsky, Cand. Sci. (Med.)
Moscow
Supplementary files
Review
For citations:
Yashin T.A., Grishina Zh.V., Parastaev S.A., Zholinsky A.V. Applicability of individual metabolites of the tricarboxylic acid cycle in athletes (A literature review). Extreme Medicine. 2025;27(2):249-256. https://doi.org/10.47183/mes.2025-288

















