Scroll to:
Alginate gels modified with natural amino acids for regenerative medicine
https://doi.org/10.47183/mes.2025-294
Abstract
Introduction. Alginate is a natural polysaccharide that shows promise as a non-toxic material for the development of hydrogel wound dressings. Natural amino acids such as arginine and lysine are important components in the synthesis of the extracellular matrix and other vital bodily processes.
Objectives. Development of alginate gels modified with arginine and lysine and compatible with dermal fibroblasts. Study the release rate of these amino acids from the gel.
Materials and methods. The study was performed using three types of aqueous solutions of 3% sodium alginate containing arginine or lysine in amounts of 5, 10, and 20% of the alginate dry weight. To assess the stability of lysine gels over time, they were incubated for 1, 3, and 7 days in 600 µL of water at room temperature. The intensity of amino acid release from the alginate gel was evaluated by the spectrophotometric method after the reaction of ninhydrin. The DF2 cell line (human dermal fibroblasts, the shared research facility “Vertebrate cell culture collection” RAS) was used as model objects. Human dermal fibroblasts were cultured on the gels obtained. The cells cultured on the gels were evaluated using optical microscopy. Statistical analysis was performed using Microsoft Excel software; Student’s t-test was used to evaluate the differences between the samples. The differences were considered statistically significant at p < 0.05.
Results. On the first day, the gel containing the minimal lysine concentration of 5% showed the amino acid release of almost 16% of its initial amount. A more prolonged incubation of the gel led to a decrease in the desorbed lysine proportion relative to the initial amount. Statistically significant differences were obtained between the samples of alginate gels with the lysine content of 5 and 20% (p < 0.05). An increase in the lysine proportion in the gel was associated with a decrease in its release, i.e., the higher concentration of lysine the gel contained, the less amount of lysin was detected after seven days of incubation. It was shown that when a gel sample with a lysine content of 5% was incubated in a large volume of water (the gel-to-water ratio of 11%), more than 40% of lysine was desorbed into water during the first hour of incubation. Therefore, lysine was 4.3% more intensively desorbed from the alginate gel compared to the arginine gel. The following pattern was established: a twofold decrease in the amount of lysine release under an increase in the gel-to-water ratio from 11% to 20%.
Conclusions. During the study, alginate gels modified with amino acids were obtained. It was found that the higher the concentration of an amino acid in the gel, the less intensively it leaves the gel. The rate of amino acid release from the gel is directly proportional to the volume of liquid in which the gel was incubated. Human dermal fibroblasts adhered better to alginate gels modified with amino acids compared to cells on alginate gels without modification. As a result of the study, gels with controlled desorption of amino acids were obtained, which promote the adhesion of human dermal fibroblasts.
For citations:
Konson V.A., Barsuk I.A., Nashchekina Yu.A. Alginate gels modified with natural amino acids for regenerative medicine. Extreme Medicine. 2025;27(2):229-234. https://doi.org/10.47183/mes.2025-294
INTRODUCTION
Wound healing is a major challenge for healthcare systems worldwide [1]. The use of wound dressings can provide a physical barrier to further infection during wound healing [2]. Ideal wound dressings should be biocompatible and biodegradable, being capable of preventing the loss of biological fluids, removing exudate, protecting the wound from pathogens, demonstrating good breathability and moisture permeability, promoting cell proliferation, and accelerating wound healing [3].
Alginate is an anionic linear block polysaccharide consisting of repeating monomeric units of (1-4)-β-D-mannuronic acid (M) and (1-4)-α-L-guluronic acid (G), capable of forming fairly user-friendly gels [4]. Alginate wound dressings are available in the form of hydrogels, foams, films, nanofibers, and sponges. Alginate dressings absorb wound fluid, resulting in the formation of gels that maintain a physiologically moist environment and thereby minimize the attachment/development of bacterial infections in the wound [5]. They can be modified to meet the requirement of chemical stability or degradation upon contact with biological fluids over a period of time. Hydrogels are used for wound healing due to their biocompatibility, as well as their ability to load and release bioactive substances of moderate porosity, high water content, and flexibility [6].
Successful wound healing is accompanied by the synthesis of extracellular matrix components. Arginine and lysine are essential amino acids that are involved in the synthesis of the extracellular matrix. Arginine is an α-amino acid with the L-form, being one of the twenty most common naturally occurring amino acids. L-arginine exhibits a number of biological activities [7]. Arginine acts as one of the key metabolites involved in the processes of nitrogen metabolism, in particular, in the ornithine cycle, characteristic of mammals. Arginine participates in the synthesis of nitric oxide (NO), which has multiple effects, ranging from anti-inflammatory to vascular effects (vasodilation) and stimulation of angiogenesis. Due to its biocompatibility and biodegradability, L-arginine is used in the development of components of numerous biomedical matrices [8]. It was shown that an overdose of L-arginine is not accompanied with the development of serious side effects, since its excess is excreted in the urine within a few hours [8]. At the same time, L-arginine is actively involved in wound regeneration, making controlled administration of this amino acid an urgent task of regenerative medicine [9].
It should be noted that lysine can also improve wound healing in the body. Lysine participates in the formation of collagen, a protein that acts as a scaffold by supporting and imparting structure to skin and bones. Lysine can act as a binder, increasing the number of new cells in the wound [10].
The process of repairing damaged tissues is quite lengthy; in this regard, it is possible to increase the efficiency of regeneration, including the synthesis of a new extracellular matrix, by dosing amino acids into the wound bed. Creating a wound dressing that exhibits not only protective and bactericidal, but also regenerating properties is an urgent task of modern regenerative medicine.
This study was aimed at developing alginate gels modified with arginine and lysine, natural amino acids, that are compatible with dermal fibroblasts and to study the release rate of these amino acids from the gel.
MATERIALS AND METHODS
Formation of gels
Three types of aqueous solutions of 3% sodium alginate (Sigma-Aldrich, USA) containing arginine or lysine amino acids (Sigma-Aldrich, USA) in amounts of 5, 10, and 20% of the alginate dry weight were prepared for experiments.
Amino acids were added in dry form followed by mixing the resulting solution thoroughly on a magnetic stirrer (Tagler MM 135H, Russia) at room temperature for 10 min. Next, an alginate solution with an amino acid was added to a 24-well plate with 300 µL of gel per well. To form a gel, the alginate in the wells was sprayed with a 10% solution of calcium chloride (CaCl2) from a spray bottle and left for 20 min at room temperature.
After gelling, the gels were washed three times with deionized water to remove amino acids that were not absorbed by the gel. The gels were then filled with a fixed volume of water (600–2500 µL) for subsequent incubation and measurement of the content of amino acids released from the gels.
To assess the stability of lysine gels over time, they were incubated for 1, 3, and 7 days in 600 µL of water at room temperature.
To assess the effect of the volume of liquid in which the gel was located on the rate of arginine release from the gel, each sample with a volume of 300 µL with an arginine content of 5, 10, and 20% by weight of alginate was kept in 600, 1200, and 2500 µL of water at room temperature for 1 h; alginate gel in relation to water occupied 33, 20, and 11%. To compare the release of arginine and lysine, the gels were incubated for 1 h in 600 µL of water at room temperature.
Determination of amino acid release from gels
The content of amino acids released from the gels was estimated by spectrophotometry (PE-5400UF spectrophotometer, Ekros, Russia). To that end, a ninhydrin reaction was carried out, during which the amino groups interacted with ninhydrin. Next, the optical density of the reaction product was measured: a violet-colored complex at a wavelength of 400 nm. This method highly very sensitive, allowing determination of amino acids in low concentrations [11].
To construct calibration graphs of the dependence of optical density on the concentration of amino acids, the following solutions were prepared: 0.2% ninhydrin solution (Sigma-Aldrich, USA) in deionized water, 0.2% lysine and arginine solutions in deionized water. Solutions of lysine and arginine at a concentration of 0.2% were used in five samples with a volume of 50, 100, 200, and 300 µL, which contained 0.1, 0.2, 0.4, and 0.6 mg of the amino acid, respectively. 250 µL of ninhydrin solution was added to each tube. The tubes were then placed in a thermostat and incubated at 100°C for 3 min. The volume of solutions in each tube was adjusted to 6 mL of deionized water and the optical density of the solutions was measured at a wavelength of 400 nm. The values obtained were used to construct calibration curves to depict the dependence of the optical density of the solution on the amount of amino acids in the solution.
The content of amino acids released from the gels in the experiments was calculated using calibration curves. To that end, 500 µL of liquid in which the gels were incubated was taken in each experiment. 250 µL of ninhydrin solution was added to tubes with liquid followed by their placement in a thermostat at 100°C for 3 min. The volume of solutions in each tube was adjusted to 6 mL of deionized water; the optical density of the solutions was measured using a spectrophotometer at a wavelength of 400 nm.
In vitro studies
The DF2 cell line (human dermal fibroblasts, INC RAS cell culture collections) was used as model objects. For experiments with cells, alginate was previously sterilized by ozone. After preparation of sterile gels with 5% amino acid, a 300 µL cell suspension was applied to gels with a diameter of 1 cm, the cell content of which was 30,000 cells per well, and incubated in a DMEM/F-12 medium (Biolot, Russia) containing 10% fetal bovine serum FBS (Gibco, USA) for 3 days. The results were recorded using an inverted microscope (Nikon eclipse, TS 100, Japan).
Statistical analysis was performed using Microsoft Excel software. Student’s t-test was used to evaluate statistically significant differences between the samples. The differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
Effect of incubation duration on the percentage of lysine release from the alginate gel
Initially, the kinetics of lysine desorption from alginate gels was investigated. Gels with a different lysine content (5, 10, and 20%) were incubated in water for 7 days. When comparing the effect of incubation duration on the amount of lysine bound to the gel, the gel with the minimum lysine concentration (5%) showed the amino acid release on the first day of almost 16% of its initial amount; the corresponding data are shown in Fig. 1. Under a longer incubation of the gel, the proportion of desorbed lysine relative to the initial amount decreased. However, statistically significant differences were found between the samples with a lysine content of 5 and 20%. This result is likely to be related to the concentration equilibrium reached in the system during prolonged incubation of gels in water.
Then, for 7 days, the amount of lysine released from the gel remain at the same level. An increase in the proportion of lysine in the gel led to a decrease in its release throughout the week: the more lysine the gel contained, the less lysine was detected after 7 days of incubation. This can be explained by the establishment of a concentration equilibrium between the gel and the solution.
The study of the effect of the volume of liquid in which the gel was incubated on the amount of lysine release found that larger volumes of water, in which the gel was incubated, were associated with a more intense desorption of amino acids from the gel. Figure 2 shows that lysine release from the gel increased with an increase in the volume of the liquid in which it was incubated.
Thus, in a gel sample with a lysine content of 5% incubated in a large volume of water (the gel-to-water ratio of 11%), more than 40% of lysine was desorbed into water during the first hour of incubation. However, a threefold increase in the proportion of gel relative to water to 33% led a decrease in the intensity of lysine release to 15%. This is approximately three times less than in the sample with the gel-to-water ratio of 11%. This case also demonstrates the pattern of reducing the amount of lysine release by half with an increase in the gel-to-water ratio from 11% to 20%. These changes can be explained by saturation of the surrounding aqueous solution with lysine during its release from the gel, which process decreased the rate of lysine desorption.
Comparison of the amount of lysine and arginine release from the gel after 1 h of incubation
There are two amino acids in the human body that are involved in metabolism and construction of new proteins. Therefore, the presence of both amino acids can promote faster tissue regeneration. Since lysine and arginine have different structural features, it should be expected that their ability to desorb from gels will also be different.
When incubating gels with both lysine and arginine in 600 µL of water, lysine was found to release out of gel more intensively than arginine (Fig. 3). Moreover, the lysine release varied from 15 to 11%, depending on its initial content, and the arginine release from 14 to 7% for gel samples with an amino acid content from 5 to 20%.
When the volume of water was increased from 600 µL to 250 µm during gel incubation, no difference in the intensity of desorption between arginine and lysine was noted.
Figures 3 and 4 show that when incubating gels in a small volume of water (600 µL), lysine left the gel faster than arginine, by an average of 4.3%. When the volume of water was increased to 2500 µL, no statistically significant differences between the desorption of arginine and lysine was observed. It should also be noted that for both amino acids, the intensity of their desorption from the alginate gel increases several times with an increase in the volume of water from 600 to 2500 µL.
In order to explain the results obtained, it is necessary to consider the structure of the molecules of these amino acids. Due to its structure, the arginine molecule is capable of imparting greater stability to proteins than lysine [10]. The guanidine group of arginine interacts with other molecules in three different directions, while the additional amino group of lysine interacts in only one direction. This feature allows arginine to form many electrostatic and hydrogen bonds and provide a stronger interaction than lysine [12].
The ionic interaction should also be taken into account. In this respect, arginine should be more stable, especially at higher pH values, due to the higher acid dissociation constant (pKa) compared with lysine [13].
It should be noted that a further increase in the amino acid content in the alginate solution prevented the formation of a gel. During the experiment, it was necessary to take into account that the viscosity of alginate depends on the acidity of the medium. Thus, according to Lee KY& Mooney, it increases with a decrease in acidity, reaching a maximum at a pH value of 3–3.5 [14].
Under physiological conditions (pH=7), the L-arginine and L-lysine amino acids have a positive charge. However, when measuring the pH of alginate solutions with these amino acids, this value turned out to be 11. Despite the high importance of pKa, especially for arginine, this could have an effect on both the amino acid charge and the stability of the alginate gel [15][16].
Human dermal fibroblasts were applied onto the surface of alginate gels followed by analysis of the gels using optical microscopy (Fig. 5). The surface of the culture plastic was used as a control. Figure 5 showed that after 3 days of culture, the cells on the plastic had a fusiform shape, characteristic of fibroblast-like cells. When applying a cell suspension to alginate gels without modification, no cells on the gels were observed: all cells migrated to the surface of the culture plastic and proliferated only on the surface of the culture vessel. When applying a cell suspension to alginate gels modified with amino acids, the cells adhered to the gels; however, their morphology was spherical. Indeed, this form is typical for cells cultured on alginate gels, since the negative charge of alginate prevents the cells from spreading, but the presence of positively charged amino acids such as lysine and arginine in the gels contributed to a sufficiently high adhesion of cells to modified alginate gels. Therefore, the introduction of amino acids into alginate gels modified with amino acids promotes cell adhesion.
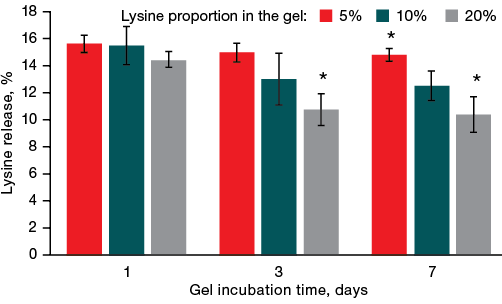
Figure prepared by the authors using their own data
Fig. 1. Kinetics of lysine release from gels
Note: * — statistical significance level p ≤ 0.05.
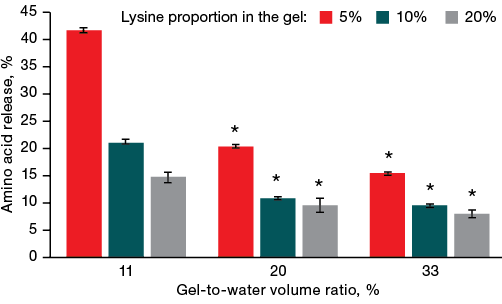
Figure prepared by the authors using their own data
Fig. 2. Effect of the gel-water ratio on the amount of lysine desorbed from the gel
Note: * — statistical significance level p ≤ 0.05.
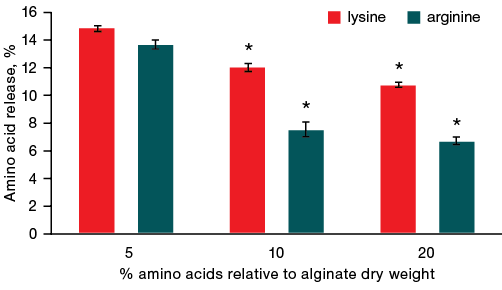
Figure prepared by the authors using their own data
Fig. 3. Dynamics of amino acid release from gels with a different ratio of amino acid and alginate after 1 h of incubation in 600 µL of liquid
Note: * — statistical significance level p ≤ 0.05.
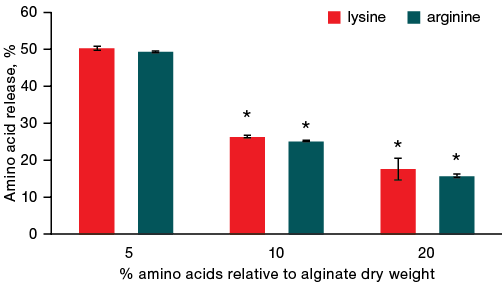
Figure prepared by the authors using their own data
Fig. 4. Dynamics of amino acid release from gels with a different ratio of amino acid and alginate after 1 h of incubation in 2500 µL of liquid
Note: * — statistical significance level p ≤ 0.05.
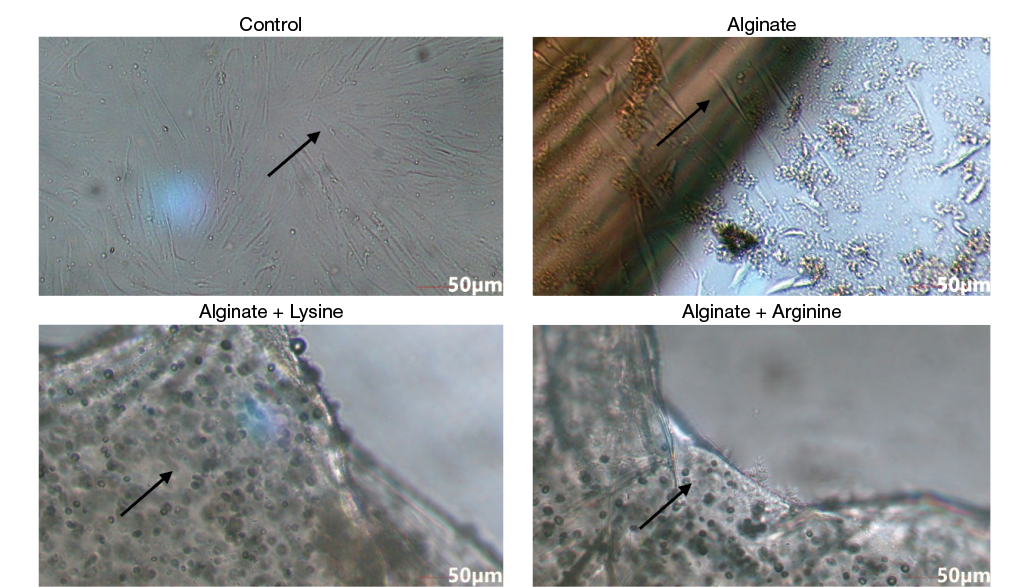
Figure prepared by the authors using their own data
Fig. 5. Optical microscopy of DF2 cells on alginate gels
Note: the arrows indicate the cells on the surface of the cup and inside the gel.
CONCLUSION
In this study, gels modified with natural amino acids — arginine and lysine — were obtained. It was found that the rate of amino acid desorption from the gel can be managed by varying the concentration of amino acids in the modified alginate gel: the higher the amino acid concentration, the less amino acid is released from the gel. In addition, the intensity of amino acid desorption can be increased by increasing the volume of water. The lysine desorption rate is higher than that of arginine from the alginate gel. The sufficiently high adhesive ability of human dermal fibroblasts to alginate gels modified with amino acids suggests that such wound dressings can be used not only as carriers for transplanted cells, but also as facilitators of migration of the patient’s own cells from the surrounding tissues bordering the wound bed.
References
1. Dong R, Guo B. Smart wound dressings for wound healing. Nano Today 2021;41:101290. https://doi.org/10.1016/j.nantod.2021.101290
2. Teoh JH, Tay SM, Fuh J, Wang CH. Fabricating scalable, personalized wound dressings with customizable drug loadings via 3D printing. J Contr Release. 2022;341:80. https://doi.org/10.1016/j.jconrel.2021.11.017
3. Liang Y, Liang Y, Zhang H, Guo B. Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci 2022;17(3):353. https://doi.org/10.1016/j.ajps.2022.01.001
4. Wang L, Sun L, Gu Z, Li W, Guo L, Ma S, et al. N-carboxymethyl chitosan/sodium alginate composite hydrogel loading plasmid DNA as a promising gene activated matrix for in-situ burn wound treatment. Bioactive Mat. 2022;15:330–42. https://doi.org/10.1016/j.bioactmat.2021.12.012
5. Song J, Chen Z, Liu Z, Yi Y, Tsigkou O, Li J, Li Y. Controllable release of vascular endothelial growth factor (VEGF) by wheel spinning alginate/silk fibroin fibers for wound healing. Mat & Des. 2021;212:110231. https://doi.org/10.1016/j.matdes.2021.110231
6. Sikareepaisan P, Ruktanonchai U, Supaphol P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr Polym 2011;83:1457. https://doi.org/10.1016/j.carbpol.2010.09.048
7. Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 2004;15(8):442. https://doi.org/10.1016/j.jnutbio.2003.11.010
8. Trischitta F, Pidala P, Faggio C. Nitric oxide modulates ionic transport in the isolated intestine of the eel, Anguilla anguilla. Comp Biochem Physiol A Mol Integr Physiol. 2007;148A(2):368. https://doi.org/10.1016/j.cbpa.2007.05.025
9. Gould AN, Candy GP. The Role of l-Arginine in Wound Healing. L-Arginine in Clinical Nutrition. Nutrition and Health. Humana Press, Cham; 2017. https://doi.org/10.1007/978-3-319-26009-9_45
10. Zhang L, Yao K, Wei J, Li G, Lin Y, Zhou YL, Yang Y. Convenient in situ synthesis of injectable lysine-contained peptide functionalized hydrogels for spinal cord regeneration. Appl Mat Today 2022;27:101506. https://doi.org/10.1016/j.apmt.2022.101506
11. Nashchekina YA, Kurdyukova KE, Zorin IM, Mikhailova NA, Bilibin AY. Spectrophotometric Evaluation of L-Lysine Concentrations in Water–Organic Solutions. Tech Phys 2018;63:1341. https://doi.org/10.1134/S106378421809013X
12. Shi J, Zhang J, Shen Y, Tang L, Zhao J, Tu J, et al. Arginine-stabilized mPEG-PDLLA (50/50) polymeric micelles of docetaxel by electrostatic mechanism for tumor-targeted delivery. Drug Delivery 2015;22(2):168–81. https://doi.org/10.3109/10717544.2013.849779
13. André I, Linse S, Mulder FA. Residue-specific pKa determination of lysine and arginine side chains by indirect 15N and 13C NMR spectroscopy: application to apo calmodulin. J Am Chem Soc. 2007;129(51):15805. https://doi.org/10.1021/ja0721824
14. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106. https://doi.org/10.1016/j.progpolymsci.2011.06.003
15. Li L, Vorobyov I, Allen TW. The different interactions of lysine and arginine side chains with lipid membranes. J Phys Chem B. 2013;117(40):11906–20. https://doi.org/10.1021/jp405418y
16. Eldin M, Kamoun M, Sofan EA. Mamdouh A,Elbayomi Smaher M. l-Arginine grafted alginate hydrogel beads: A novel pH-sensitive system for specific protein delivery. Arab J Chem. 2015;8:355. https://doi.org/10.1016/j.arabjc.2014.01.007
About the Authors
V. A. KonsonRussian Federation
Valentina A. Konson
St. Petersburg
I. A. Barsuk
Russian Federation
Ilya A. Barsuk
Moscow
Yu. A. Nashchekina
Russian Federation
Yuliya A. Nashchekina, Cand. Sci. (Biol.)
St. Petersburg
Supplementary files
Review
For citations:
Konson V.A., Barsuk I.A., Nashchekina Yu.A. Alginate gels modified with natural amino acids for regenerative medicine. Extreme Medicine. 2025;27(2):229-234. https://doi.org/10.47183/mes.2025-294

















