Scroll to:
Potentially significant markers of immune system and blood clotting residual disorders in athletes with a history of COVID-19
https://doi.org/10.47183/mes.2025-297
Abstract
Introduction. The increase in the number of patients with post-acute sequelae of COVID-19, including athletes, requires improved diagnostic methods for the long-term effects of this disease. The study of changes in the blood-clotting sequence and immune regulation in a limited but homogeneous group of athletes (intense physical activity and careful health monitoring) with post-acute sequelae of COVID-19 can contribute to identification of reliable diagnostic and prognostic biomarkers of this condition.
Objective. A comparative analysis of the cytokine profile and hemostasis system features as promising prognostic markers for the diagnosis of post-acute sequelae of COVID-19 in athletes.
Materials and methods. 60 athletes (24 men and 36 women, average age 20.8 ± 1.86 years) were examined. All participants were divided into two groups: group 1 — 40 athletes who had suffered from coronavirus infection; group 2 (control) — 20 athletes who had not had COVID-19. The athletes specialized in various sports: figure skating, rhythmic gymnastics, athletics, rugby, and wrestling. To assess the residual effects of COVID-19, biochemical parameters were studied in all participants: alanine aminotransferase activity, aspartate aminotransferase, C-reactive protein level, troponin-I level; hemostasis parameters: prothrombin time (PT), prothrombin index (PTI), activated partial thromboplastin time (aPTT), international normalized ratio (INR), D-dimer; immune status indicators: interleukins-6, -8, -10 (IL-6, -8, -10, respectively), tumor necrosis factor-α (TNF-α). Statistical data processing was carried out using the Statistica 10 software.
Results. An increase in clotting time was revealed in terms of aPTT, prothrombin time, international normalized ratio, and a decrease in the prothrombin index (p < 0.05). Statistically significant differences in the functional state of the immune system were also found: an increase of IL-8 from 2.5 to 7.42 times [3.08; 9.96] pg/mL and IL-10 from 2 to 5.08 times [2.93; 6.66] pg/mL compared to similar indicators in athletes who had not suffered from COVID-19 — 3.05 [1.86; 8.15] pg/mL and 2.53 [1.0; 5.68] pg/mL (p < 0.05) in the group of athletes who had undergone COVID-19. A direct correlation was established between an increase in IL-8 levels and an increase in PT (rs = 0.355; p < 0.05) and INR (rs = 0.420; p < 0.05) in athletes who had undergone a coronavirus infection. At the same time, a negative association was found between an increase in IL-8 levels and a decrease in PTI (rs = –0.323; p < 0.05).
Conclusions. Higher levels of activating cytokines and lower values of parameters of the anti-inflammatory immune system indicate residual dysregulatory phenomena in the immune system in post-acute sequelae of COVID-19. The revealed relationships between the coagulation profile and the components of the immune response allow these relationships to be considered as possible diagnostic markers of residual phenomena after coronavirus infection. The data obtained confirm the validity of IL-8 and IL-10 indicators as potential markers of residual disorders after COVID-19 in athletes. However, these findings should be verified on larger samples, where the observed ratios may show a different dynamics.
For citations:
Efimov P.V., Tarasova M.S., Zholinsky A.V., Parastaev S.A. Potentially significant markers of immune system and blood clotting residual disorders in athletes with a history of COVID-19. Extreme Medicine. 2025;27(2):243-248. https://doi.org/10.47183/mes.2025-297
INTRODUCTION
The term “post-acute sequelae of COVID-19” (PASC) was proposed in February 2020 to describe the residual manifestations detected in individuals with a history of SARS-CoV-2 infection. Further, in October 2020, this syndrome received a separate ICD-10 code: U09.9 — post COVID-19 condition, unspecified.
During the development of PASC, symptoms that cannot be explained by an alternative diagnosis appear, on average, three months after the onset of the disease. At the moment, the literature proposes no model for the diagnosis of PASC for both the general population, whose degree of motor inertia has increased significantly over the years of the pandemic, and athletes, whose share in the global population is extremely insignificant [1]. However, although the positive effect of regular physical activity on the body’s resistance to infectious agents is generally recognized, which is essential during periods of epidemiological outbreaks, the effects of excessive physical exertion, characteristic of high-performance sports, are not that unambiguous. Thus, the so-called “open window effect” occurs during certain stages of one-year training, expressed in an increase in the body’s susceptibility to infectious diseases after significant physical exertion [2].
The PASC clinical manifestations are highly diverse: a total of 55 long-term symptoms associated with COVID-19 have been described. Most of them correspond to clinical symptoms or syndromes of the central nervous system and the mental health, respiratory, cardiovascular, immune, digestive systems, etc. A meta-analysis of studies (n = 15), which included characterization of the PASC signs, showed that up to 80% of patients who have suffered COVID-19 experience long-term consequences in the form of mono-symptoms and their associations [3]. However, other values were obtained for the cohort of athletes. Thus, the study [4] examining the long-term effects of the disease among 11,518 athletes of various skill levels found that the incidence of PASC persistent symptoms was only 8.3%. The tendency towards a milder (often asymptomatic) course of COVID-19 in a cohort of athletes with a lower probability of complications, including viral pneumonia, than in the general population, can be considered as a probable cause of such pronounced differences.
In addition to routine markers, diagnostic search programs for the sports contingent were advised to include an assessment of laboratory parameters of cardiac function, hormonal status, immune response, and the coagulation system. In most cases, the values of the studied parameters remain within the reference ranges; however, long-term deviations are also possible, more often of minor severity [4].
Data on the immune system parameters and coagulation profile in patients with the post-acute sequelae of COVID-19 are heterogeneous. A recent meta-analytical study [5], which covered 23 publications, showed that patients with suspected PASC are more likely to have elevated levels of leukocytes, C-reactive protein (CRP), and D-dimer; however, the prognostic effect of deviations was assessed as insignificant. At the same time, the authors [6] proposed to consider the D-dimer and CRP as some non-cytokine markers, the values of which increase in patients infected with SARS-CoV-2. At the same time, more pronounced differences in the D-dimer level are associated with concomitant pathology revealed by imaging and functional testing methods [7].
In addition, after COVID-19, there may be a slight increase in the activity of other components of the blood-clotting sequence [5], as well as changes in the effector link of the immune response, namely increased activation of T cells, as evidenced by the level of the soluble interleukin-2 receptor [8].
The study [9] reported changes in the immunoregulation system, which were multidirectional in some cases. Thus, it was noted that the levels of interleukin-6 (IL-6), often considered as the leading indicator of proinflammatory cytokine activity, were higher in patients with PASC compared to practically healthy patients and individuals without long-term consequences from COVID-19. The authors in [10] proposed this cytokine, as well as tumor necrosis factor alpha (TNF-α), to be considered as potential predictors of COVID-19 severity in acutely infected patients without concomitant diseases. It should be noted that IL-6 can be actively produced in muscle tissue along with other myokines (myostatin, insulin-like growth factor, fibroblast growth factor, etc.) associated with the level of physical activity [11]. This fact makes it difficult to validate IL-6 levels as a PASC marker in the athlete cohort due to its significant dependence on the level of training and competitive activity.
At the same time, the interleukin-8 (IL-8) level, which exhibits proinflammatory activity, is associated with the COVID-19 severity in the acute period and may be directly involved in the pathogenetic pathways of the formation of post-acute sequelae of COVID-19 [12].
One of the main anti-inflammatory cytokines, interleukin-10 (IL-10), has a pleiotropic effect on the immune response. The researchers in [13] emphasized that IL-10 is a predictor of the severity and mortality of patients with acute infection and residual COVID-19 effects. In this context, IL-10 can act as an endogenous anti-inflammatory component secreted by damaged tissues in response to a hyperinflammatory state, which also allows this indicator to be assumed as a PASC marker.
The growing number of patients with post-acute sequelae of COVID-19, including athletes with a high probability of latent post-infectious myocardial damage, substantiates the need to optimize approaches to diagnosing the long-term consequences of COVID-19. Research into the dynamics of changes in hemostasis and immune regulation in the target group of athletes with PASC, which may be insignificant in number but fairly homogeneous in terms of the characteristics taken into account (significant physical activity and targeted/constant monitoring of health status), is likely to contribute to establishing valid diagnostic and prognostic markers of this condition.
In this study, we set out to compare the features of the cytokine profile and the hemostasis system as promising prognostic markers of post-acute sequelae of COVID-19 in athletes.
MATERIALS AND METHODS
In total, 60 athletes were examined as part of the research work. The study included 24 male and 36 female athletes, with an average age of 20.8 ± 1.86 years. All participants were divided into two groups: group 1 — 40 athletes who had suffered from coronavirus infection (according to medical records); group 2 (control) — 20 athletes who had not suffered from coronavirus infection (according to medical records). All athletes were at the stages of improvement and possessed higher sports skills, specializing in various sports: figure skating (n = 16), rhythmic gymnastics (n = 15), athletics (n = 10), as well as rugby (n = 10) and wrestling (n = 9). The study was conducted during the preparatory period of the training process.
The inclusion criteria were age from 18 to 45 years, history of coronavirus infection, informed voluntary consent of the subjects. The exclusion criteria were the patient’s refusal to participate in the study.
For residual COVID-19 estimation, a venous sampling was performed in all study participants. Blood draw was performed in 30 sportsmen in 2023 (19.05.2023–22.09.2023) and in 30 sportsmen in 2024 (14.06.2024–10.07.2024). Venous sampling was performed following an overnight fast from the peripheral vein with the help of a vacuum system in tubes with anticoagulant. The following parameters were evaluated: biochemical — alanine aminotransferase (ALT), aspartate aminotransferase (AST), C-reactive protein (CRP), troponin-I level (highly sensitive method); hemostasis parameters — prothrombin time (PT), prothrombin index (PTI), activated partial thromboplastin time (aPTT), international normalized ratio (INR), D-dimer; immune status indicators — interleukins-6, -8, -10 (IL-6, -8, -10, respectively), tumor necrosis factor-α (TNF-α).
The immune parameters were evaluated by enzyme-linked immunosorbent on a Real R microplate photometer (Vector-Best, Russia) and tubes with plasma and EDTA Na2 solution (6.7%) with Tween-20. To evaluate the levels of troponin-I, C-reactive protein, ALT, AST, and coagulation profile, a Cobas 501c analyzer (Roche, Germany) was used along with a tube with plasma treated with Kz-ETDA. The assay was carried out using the following reagent sets: ALT and AST — “ALT” and “AST” in accordance with IFCC without pyridoxal phosphate activation, CRP — “CRP4” Tina-quant C-Reactive Protein IV, troponin-I — “Elecsys Troponin I” (Roche Diagnostics GmbH, Germany); aPTT — “Pathromtin SL”, PTB/PTI — “Thromborel S”, D-dimer — “INNOVATION D-Dimer” (Siemens Healthcare Diagnostics, Germany).
The STATISTICA 10 software was used for statistical data processing. The samples were checked for compliance with the normal distribution law by Shapiro–Wilk test statistics. The variables were represented as the median (Me) and the interquartile range [Q25; Q75]. In the presence of a normal distribution, Student’s t-test was calculated for unrelated samples. When the distribution was different from normal, the nonparametric Mann–Whitney U-test was used for comparative intergroup analysis. Spearman’s rank correlation coefficient (rs ) was used to identify intragroup correlations. The statistical significance level was assumed to be 0.05.
RESULTS AND DISCUSSION
During the study, athletes from group 1 showed a statistically significant increase in clotting time: an increase in aPTT to 36.6 [ 34.3; 39.9] s, aPTT of 12.2 [ 11.5; 12.8] s and INR of 1.11 [ 1.08; 1.16], a decrease in PTI of 89.9 [ 85.6 93.3] % compared with the indicators of athletes from the second group — 34.9 [ 32.8; 35.7] s, 11.5 [ 11.1; 11.9] s, 1.06 [ 1.02; 1.11], 95.9 [ 88.5; 101,4] % (p < 0.05), respectively. The relevant data is shown in Fig. 1.
At the same time, the direction of the changes observed contradicts the literature data that people with a history of COVID-19 are more likely to have a procoagulant condition. The affinity of SARS-CoV-2 to angiotensin converting enzyme 2 (ACE2) receptors expressed on endothelial cells triggers a cascade of events provoking endothelial damage, which leads to dysregulation of thrombo-inflammatory reactions characterized by an increased release of von Willebrand factor, impaired fibrinolysis, and subsequent hypercoagulation [14]. The group of researchers [15], who examined the first patients hospitalized in Wuhan, found elevated levels of aPTT, PTV, and D-dimer. Subsequently, a tendency to hypercoagulation was also noted in a number of studies in patients who had suffered from COVID-19 [16-18]. It is likely that the level of physical activity and fitness of athletes contributes to a less pronounced effect of SARS-CoV-2 on the hemostasis system.
No statistically significant intergroup difference was found in the athletes in terms of ALT, AST, D-dimer, TNF-α parameters, probably because the study included the athletes without significant cardiorespiratory disorders. No comparative analysis was performed for the highly sensitive troponin-I index due to the low variance of values.
When considering the functional state of the immune system, statistically significant differences were obtained for IL-8 and IL-10 levels, indicating disorders in the form of an imbalance of regulatory cytokine activity with the predominance of pro-inflammatory effects. Thus, athletes with a history of COVID-19 showed a 2.5-fold increase in IL-8 to 7.42 [ 3.08; 9.96] pg/mL and IL-10 2-fold to 5.08 [ 2.93; 6.66] pg/mL compared to similar indicators in athletes without a history of COVID-19 — 3.05 [ 1.86; 8.15] pg/mL and 2.53 [ 1.0; 5.68] pg/mL (p < 0.05), respectively (Fig. 2), which indicates a relative lack of activation of the anti-inflammatory immune system in athletes who have undergone COVID-19.
The data obtained on the level of cytokines in post-acute sequelae of COVID-19 are consistent with the data presented in the scientific literature. Thus, the study [19] found a significant increase in the levels of most regulatory chemokines, including IL-8 and IL-10, in patients with COVID-19. The researchers in [20] conducted an analysis of the cytokine profile in patients with PASC within 2–3 months after COVID-19 and detected high levels of cytokines IL-8 and IL-10. It was shown that their average levels in those with the decease history were twice higher than in healthy individuals. In our study, however, no significant changes in IL-6 levels were found in athletes who had undergone COVID-19 compared to athletes without such a history, the content of which is directly associated with the development of post-COVID-19 residual disorders [21]. It is likely that in the cohort of athletes, taking into account the level of physical activity, IL-8 and IL-10 are more correlated with the development of post-COVID disorders.
In the course of the study, we identified the dependence of coagulative hemostasis parameters and immune disorders in athletes who had a history of coronavirus infection (Table 1).
Thus, a weak direct correlation was established between an increase in IL-8 levels and an increase in PT (rs = 0.355; p < 0.05) and INR (rs = 0.420; p < 0.05). At the same time, a moderate negative association was found between an increase in IL-8 levels and a decrease in PTI (rs = –0.323; p < 0.05). Given that such correlations have not been found in athletes who have not had COVID-19, the ratio of IL-8 levels with various coagulation profiles can probably be considered as a potential marker of residual COVID-19 effects.
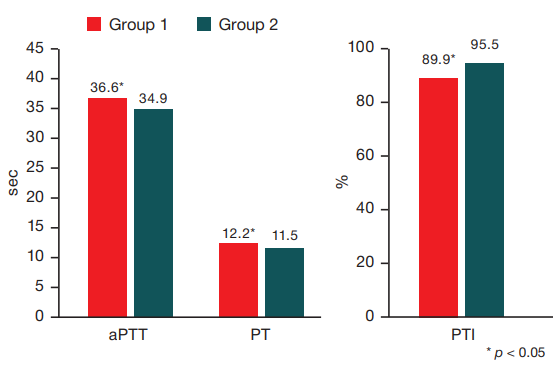
Figure prepared by the authors using their own data
Fig. 1. Coagulation profile changes among the study participants
Note: aPTT — activated partial thromboplastin time, PT — prothrombin time, PTI — prothrombin index, INR — international normalized ratio.
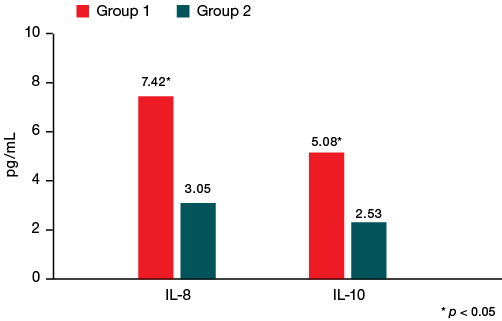
Figure prepared by the authors using their own data
Fig. 2. Changes in some indicators of immune status among the study participants
Note: IL-8 — interleukin-8; IL-10 — interleukin-10.
Table 1. Correlation analysis of the studied indicators in the group of athletes with a history of COVID-19
|
Variables |
IL-8 |
IL-10 |
aPTT |
PT |
PTI |
INR |
|
IL-8 |
-0.087 |
0.112 |
0.355 |
-0.323 |
0.420 |
|
|
IL-10 |
-0.087 |
0.136 |
-0.184 |
0.214 |
-0.056 |
|
|
aPTT |
0.112 |
0.136 |
0.065 |
0.115 |
0.099 |
|
|
PT |
0.355 |
-0.184 |
0.065 |
-0.920 |
0.871 |
|
|
PTI |
-0.323 |
0.214 |
0.115 |
-0.920 |
-0.770 |
|
|
INR |
0.420 |
-0.056 |
0.099 |
0.871 |
-0.770 |
Table prepared by the authors using their own data
CONCLUSION
A comparative analysis of the functional state of the coagulative hemostasis system and the immune profile in athletes who have had coronavirus infection revealed multi-vector changes in the parameters of the coagulation system in the form of an increase in blood clotting time according to the main parameters of the coagulation profile of aPTT, PT, INR, as well as a decrease in PTI.
Athletes with a history of coronavirus infection are characterized by a relative predominance of pro-inflammatory cytokine activity over anti-inflammatory activity, which is manifested by a more pronounced increase in the level of IL-8 than IL-10, identifying an imbalance in the regulatory link of the immune response.
In the course of the correlation analysis, a direct moderate relationship was established between an increase in IL-8 levels and an increase in PTV and INR, as well as a negative correlation between an increase in IL-8 levels and a decrease in PTI, which partially confirms the validity of IL-8 and IL-10 indicators as potential markers of residual disorders after COVID-19 in athletes. The associations between the parameters of the coagulation hemostasis system and the immune profile allow us to consider their ratios as diagnostic criteria for residual effects in athletes who have undergone COVID-19.
References
1. Marques A, Henriques-Neto D, Peralta M, Martins J, Demetriou Y, Schönbach DMI, de Matos MG. Prevalence of Physical Activity among Adolescents from 105 Low, Middle, and High-income Countries. Int. J. Environ. Res. Public Health. 2020;17:3145. https://doi.org/10.3390/ijerph17093145
2. Slivin AV, Efimov PV, Zorenko AV, Kupeev MV, Yashin TA, Yadgarov MY, et al. On the use of glutamine-containing specialty foods in sports. Sports medicine: research and practice. 2021;11(4):57–68 (In Russ.). https://doi.org/10.47529/2223-2524.2021.4.8
3. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. https://doi.org/10.1038/s41598-021-95565-8
4. Lemes IR, Smaira FI, Ribeiro WJD, et al. Acute and post-acute COVID-19 presentations in athletes: a systematic review and meta-analysis. Br J Sports Med. 2022;56(16):941–7. https://doi.org/10.1136/bjsports-2022-105583
5. Yong SJ, Halim A, Halim M, et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers. Reviews in medical virology, 33(2):e2424. https://doi.org/10.1002/rmv.2424
6. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–44. https://doi.org/10.1016/S2213-2600(20)30404-5
7. Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–8. https://doi.org/10.1136/thoraxjnl-2020-215818
8. Grazioli S, Tavaglione F, Torriani G, et al. Immunological Assessment of Pediatric Multisystem Inflammatory Syndrome Related to Coronavirus Disease 2019. J Pediatric Infect Dis Soc. 2021;10(6):706–13. https://doi.org/10.1093/jpids/piaa142
9. Queiroz MAF, Neves PFMD, Lima SS, et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front Cell Infect Microbiol. 2022;12:922422. https://doi.org/10.3389/fcimb.2022.922422
10. Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43. https://doi.org/10.1038/s41591-020-1051-9
11. Barbalho SM, Prado Neto EV, De Alvares Goulart R, et al. Myokines: a descriptive review. J Sports Med Phys Fitness. 2020;60(12):1583–90. https://doi.org/10.23736/S0022-4707.20.10884-3
12. Bekbossynova M, Tauekelova A, Sailybayeva A, et al. Unraveling Acute and Post-COVID Cytokine Patterns to Anticipate Future Challenges. J Clin Med. 2023;12(16):5224. https://doi.org/10.3390/jcm12165224
13. Carlini V, Noonan DM, Abdalalem E, et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023;14:1161067. https://doi.org/10.3389/fimmu.2023.1161067
14. Obeagu EI, Tukur M, Akaba K. Impacts of COVID-19 on hemostasis: coagulation abnormalities and management perspectives. Ann Med Surg. 2024;86(10):5844–50. https://doi.org/10.1097/MS9.0000000000002237
15. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. https://doi.org/10.1016/s0140-6736(20)30211-7
16. Jerah AA, Farasani A, Abu-Tawil H, et al. Unveiling coagulation dysfunction in patients with COVID-19: a retrospective analysis. J Med Life. 2024;17(9):886–91. https://doi.org/10.25122/jml-2024-0166
17. Ikiz F, Ak A. Investigation of the relationship between coagulation parameters and mortality in COVID-19 infection. Blood Sci. 2024;6(2):e00191. https://doi.org/10.1097/bs9.0000000000000191
18. Sekulovski M, Mileva N, Vasilev GV, et al. Blood Coagulation and Thrombotic Disorders following SARS-CoV-2 Infection and COVID-19 Vaccination. Biomedicines. 2023;11(10):2813. https://doi.org/10.3390/biomedicines11102813
19. Gomes SMR, Brito ACS, Manfro WFP, et al. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients. PLoS One. 2023;18(4):e0283983. https://doi.org/10.1371/journal.pone.0283983
20. Zhdanova EV, Rubtsova EV, Kostolomova EG. Clinical and immunological characteristics of post-COVID syndrome. Bulletin of Siberian Medicine. 2024;23(2):46–54 (In Russ.). https://doi.org/10.20538/1682-0363-2024-2-46-54
21. Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6):100663. https://doi.org/10.1016/j.xcrm.2022.100663
About the Authors
P. V. EfimovRussian Federation
Pavel V. Efimov
Moscow
M. S. Tarasova
Russian Federation
Mariya S. Tarasova
Moscow
A. V. Zholinsky
Russian Federation
Andrey V. Zholinsky, Cand. Sci. (Med.)
Moscow
S. A. Parastaev
Russian Federation
Sergey A. Parastaev, Dr. Sci. (Med.), Professor
Moscow
Supplementary files
Review
For citations:
Efimov P.V., Tarasova M.S., Zholinsky A.V., Parastaev S.A. Potentially significant markers of immune system and blood clotting residual disorders in athletes with a history of COVID-19. Extreme Medicine. 2025;27(2):243-248. https://doi.org/10.47183/mes.2025-297

















