Scroll to:
Analysis of factors influencing apoptotic processes during formation of long-term health effects of severe acute poisoning with neurotropic toxicants
https://doi.org/10.47183/mes.2024-247
Abstract
Background. The nervous system damage caused by neurotoxicants is characterized by various morphological changes, manifested mainly by dystrophic and necrotic processes. The key mechanisms of post-intoxication asthenia pathogenesis, determined by the specifics of the toxicant, involve activation of apoptosis, trophic disorder, lipid peroxidation (LPO), neuropeptide regulatory insufficiency, as well as cerebrospinal fluid dynamics disorders.
Objective. Quantification of the contribution of apoptosis, oxidative stress, and neurotrophin regulation processes to the formation of long-term health consequences of severe acute poisoning with neurotropic toxicants.
Material and methods. Experimental studies were performed in male rats. The following toxicants were used: phenylcarbamate (1.6 mg/kg bw), methanol (11.5 g/kg bw), lead acetate (300 mg/kg bw). The period of formation of long-term health effects was 30 days. The level of apoptosis of the brain temporal cortex neurons was evaluated by the TUNEL method. The identification of blood plasma neurospecific proteins was carried out by the ELISA method. Evaluation of LPO and antioxidant system was carried out by standard biochemical methods.
Results. Exposure to the substances caused the signs of toxic effects in rats on days 1–2. The maximum severity of poisoning with phenylcarbamate was on the first day, while the maximum severity of poisoning with methanol and lead acetate was manifested on the second day. By days 5–7, the survived animals showed a normalization in the status regardless of the toxicant. On day 30, violations were detected, the totality of which allowed the survived animals to be divided into subgroups according to the manifestation of functional signs of long-term health effects of acute poisoning.
Conclusions. The formation of long-term health effects of severe acute poisoning with the studied neurotoxicants was shown to be associated with an increase in the number of TUNEL positive neurons and a decrease in the S100 protein serum concentration. Lipid peroxidation in brain tissues during the specified period did not play a significant role in apoptosis activation.
Keywords
For citations:
Shustov E.B., Melnikova M.V., Masterova K.V., Ostrov V.F., Zolotoverkhaja E.A., Kubarskaya L.G., Tanayants K.O., Kozlov A.A., Sokolova Yu.O., Potapov P.K. Analysis of factors influencing apoptotic processes during formation of long-term health effects of severe acute poisoning with neurotropic toxicants. Extreme Medicine. 2025;27(1):5-14. https://doi.org/10.47183/mes.2024-247
INTRODUCTION
The long-term period following severe acute poisoning with neurotropic toxicants is characterized by the development of mainly nonspecific manifestations of the psychoorganic syndrome, post-intoxication and cerebrogenic asthenia, toxic polyneuropathies. Toxic damage to the nervous system, as well as to other body tissues, involves various morphological changes at the cellular and tissue levels and is manifested by various dystrophic and necrotic processes [1–2]. The use of light microscopy makes it possible to establish the presence of neuronal apoptosis, which is a consequence of most toxic lesions of the nervous system [3]. Toxic damage to brain tissue is characterized by small foci of necrosis, often elective, in which only individual tissue elements are damaged (some of them remain); such foci are manifested by rarefaction of the neuropil and gliopenia. The morphological basis of the psychoorganic syndrome in neurointoxication consists in the death of neurons and glial cells associated with both the direct toxic effect of xenobiotics and the induction of apoptotic processes [4]. The key mechanisms behind the pathogenesis of post-intoxication asthenia, determined by the specifics of the toxicant, involve activation of apoptosis, trophic disorder, lipid peroxidation (LPO), neuropeptide regulatory insufficiency, and cerebrospinal fluid dynamics disorders. Toxic neuropathies are manifested by segmental demyelination (toxic myelopathy) and axonal degeneration (toxic distal axonopathy).
The nonspecific mechanisms of toxic action characteristic of all neurotropic xenobiotics include inhibition of enzyme activity due to the blockade of sulfhydryl, carboxyl, amino, and other functionally active structural groups in peptides and proteins; formation of oxidative stress with subsequent activation of LPO processes1; mitochondrial inhibition; calcium homeostasis disorder; excitotoxicity; proinflammatory cytokine expression, inflammatory process induction in nervous tissue; haptenic modification of proteins with their acquisition of antigenic properties and induction of autoimmune damage mechanisms; inhibition of neurotrophin release, neurogenesis and gliogenesis in combination with suppression of proliferation and differentiation of new neurons and gliocytes; increased permeability of the blood-brain barrier; increased processes of cell apoptosis of the central and peripheral nervous system2 [5–8].
Taking all the above mentioned into account, a deeper understanding of the long-term health effects of severe acute poisoning with neurotropic toxicants is required. In this connection, the present study aims to assess the quantitative contribution of apoptosis, oxidative stress, and neurotrophin regulation processes to the formation of long-term health consequences of severe acute poisoning with neurotropic toxicants.
MATERIALS AND METHODS
The experimental study involved 53 male outbred rats with a body weight (bw) of 180–220 g (baseline), received from the Kurchatov Institute Research Center — Rappolovo Nursery (Leningrad Region, Russia).
Long-term effects were simulated by a single administration of a neurotropic toxicant to laboratory animals at a dose of LD50. The period of formation of long-term consequences was 30 days. The following were used as toxicants:
- phenylcarbamate, a reversible acetylcholinesterase inhibitor, synthesized in the Golikov Research Center of Toxicology [11];
- methanol (Vecton, Russia), an organic solvent that implements its toxic effect by damaging cell membranes;
- lead acetate (Reahim, Russia), an organic heavy metal salt that suppresses the activity of various enzymes.
Laboratory animals were divided into four groups:
- group 1 or C (Control) (n = 8), intragastric administration of 0.5 mL of saline solution;
- group 2 or PC (Phenylcambamate) (n = 15), intraperitoneal administration of an 0.1% aqueous solution at a dose of 1.6 mg/kg bw;
- group 3 or M (Methanol) (n = 15), intragastric administration of a 75% aqueous solution at a dose of 5 g/kg bw;
- group 4 or LA (Lead acetate) (n = 15), intraperitoneal administration of a 5% aqueous solution at a dose of 300 mg/kg bw.
The signs of toxic effects identified as a result of observation were evaluated in points according to the scale of signs of intoxication [10]. The dynamics of the animals’ body weight, their consumption of feed and water were determined weekly.
To assess the formation of the phase of long-term consequences, neurophysiological testing of animals was performed on days 7, 15, 28 by such tests as open field (VideoMot 2, TSE, Germany), rotating rod (Rota-Rod Treadmills for rats 7700-7750, Ugo Basile, Italy), grip strength (Gripstrengthmeter 303500 series, TSE, Germany), sensory reactions tests (Startle Response System, TSE, Germany), assessment of the conditioned passive avoidance reflex (CPAR) (PACS-30, Columbus Instruments, USA).
On day 28, blood and brain tissue samples were collected from the survived animals (n = 32) for subsequent examination.
The determination of neurospecific proteins (neurospecific enolase NSE, brain neurotrophic factor BDNF, basic myelin protein MBP and calcium-binding protein S100) in animal blood plasma was carried out by enzyme immunoassay using commercial kits (Cloud-Clone Corp., USA) according to the manufacturer’s instructions. Indicators of lipid peroxidation (diene conjugates of DC, malonic dialdehyde MDA); the antioxidant system of AOS (reduced glutathione GSH, activity of glutathione transferase GST, glutathione peroxidase GP, superoxide dismutase SOD), as well as energy metabolism (activity of glucose-6-phosphate dehydrogenase G6PDH) were determined in the homogenate of brain tissue, as described in [11].
The activity of apoptotic processes was assessed by determining the number of TUNEL-positive (terminal deoxynucleotidyl transferase dUTP nick end labeling) cells in the temporal cortex of rats. The choice of this area of the cortex was determined by the peculiarities of its cytoarchitectonics (high density of neurons, radial divergence of cortical columns, presence of eight types of interneurons and projections from the auditory, statokinetic, gustatory and olfactory analyzers, thalamus, their high sensitivity to hypoxia, frequent dysplasia and the ability to epileptogenesis). Apoptotic TUNEL cells were counted on 4–5 slices of the studied brain area using a commercial Elabscience® E-CK-A320 kit “TUNEL In Situ Apoptosis Kit (Green, FITC), One-step TUNEL In Situ Apoptosis Kit”. Images of brain slices were obtained using a Nikon Eclipse 80i microscope with a Nikon DS-Fi1c color camera at a magnification of ×100 for analysis and ×200 for photographic materials at a resolution of 1280×960 pixels using the NIS-Elements AR 4.20.00 software application. Apoptotic TUNEL cells were counted on 4–5 slices of the studied area of the brain of each animal.
Statistical processing of the experimental data obtained was carried out in the MS EXCEL spreadsheet processor using the Data Analysis application software and the specialized statistical analysis software Statistica v.10. The methods of frequency, variance, factor, and correlation analysis were used. The significance of differences between the groups was assessed using the rank-sum test (White), the Fisher’s exact probability test for frequency analysis, the F-criterion of the analysis-of-variance (ANOVA).
RESULTS
Observation of animals during the acute intoxication period showed that toxic effects signs (according to the sum of points characterizing the features of appearance, muscle tone, motor activity, and reaction to gripping) were detected on 1–2 days, with a rapid normalization of the condition by days 5–7 in the survived animals (Fig. 1).
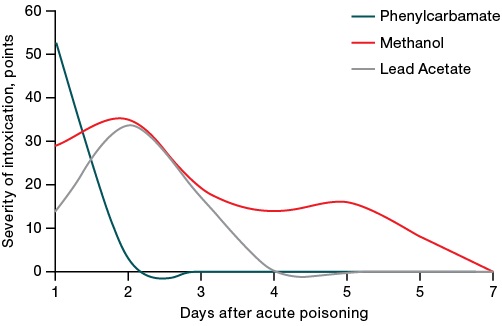
Figure prepared by the authors based on their own data
Fig. 1. Dynamics of intoxication severity after administration of LD50 toxicants
The clinical picture of acute phenylcarbamate intoxication was dominated by signs of seizures and ataxia. Methanol poisoning led to a decreased muscle tone and motor activity, impaired coordination of movements and posture, decreased sensitivity and reflexes. Under lead acetate intoxication, the enteropathogenic symptom complex, irritation of the peritoneum, impaired motor activity and respiration were observed. At the same time, different toxicants were characterized by a different rate of increase and decrease in the symptoms of intoxication (the fastest and most pronounced manifestation of intoxication, an intensive decrease for phenylcarbamate, a slower decrease for methanol). Lead acetate was characterized by a slower increase in poisoning symptoms, which is associated with the peculiarities of its absorption in the body.
During dynamic observation of animals, no statistically significant differences were found between the control group and the groups of animal survivors of acute intoxication in terms of such indicators as body weight, feed intake, paw grip strength, retention time on a rotating rod, as well as in terms of individual indicators of the neurophysiological techniques used (locomotor activity, emotional lability, aggressiveness, grooming in the open field test; locomotor activity and staying in the illuminated sector during the CPAR test; indicators of reflex response to harsh sound and latency characteristics in the Startle Response System test). Therefore, these indicators were not reflected in the tables and figures following below.
On day 30 of the study, signs of impaired ingestive (feeding and drinking) behavior, reproduction of CPAR, coordination of movements, inhibition in polysynaptic reflex circuits, depletion of activation influences were revealed (Table 1). The totality of these signs allowed the animal poisoning survivors to be divided into subgroups according to the level of formation of functional signs of the long-term health consequences of acute poisoning.
Table 1. Dynamics of neurophysiological parameters in the post-intoxication period
|
Indicator, test |
Group |
Days after intoxication |
|||
|
7 |
15 |
21 |
28 |
||
|
Water consumption, mL/day # |
Control |
26.9 |
25.6 |
27.5 |
28.1 |
|
Phenylcarbamate |
32.8* |
32.2 |
33.3* |
36.8* |
|
|
Methanol |
32.0* |
35.5* |
21.1 |
20.0* |
|
|
Lead Acetate |
24.0 |
23.0 |
21.1 |
20.0* |
|
|
Search and research activity, units |
Control |
9.2 ± 1.7 |
8.9 ± 1.6 |
6.0 ± 1.5 |
4.9 ± 1.0 |
|
Phenylcarbamate |
11.4 ± 1.1 |
10.2 ± 1.2 |
6.2 ± 1.3 |
5.6 ± 1.3 |
|
|
Methanol |
2.8 ± 0.7* |
3.1 ± 0.8* |
4.5 ± 0.4 |
4.9 ± 1.0 |
|
|
Lead Acetate |
14.6 ± 0.9 |
12.4 ± 2.0 |
8.2 ± 2.0 |
9.2 ± 1.2* |
|
|
Duration of stay in a dark cell, s |
Control |
29 ± 15 |
- |
- |
4 ± 3 |
|
Phenylcarbamate |
75 ± 11* |
- |
- |
33 ± 13* |
|
|
Methanol |
35 ± 14 |
- |
- |
24 ± 13 |
|
|
Lead Acetate |
35 ± 23 |
- |
- |
40 ± 2.5* |
|
|
Treadmill, proportion of completed the test, % |
Control |
100 |
100 |
100 |
100 |
|
Phenylcarbamate |
89 |
100 |
78 |
33 |
|
|
Methanol |
100 |
0♦ |
44 |
44 |
|
|
Lead Acetate |
100 |
30 |
70 |
0♦ |
|
|
Response to sound, amplitude, units |
Intact |
76 ± 34 |
123 ± 63 |
247 ± 113 |
|
|
Phenylcarbamate |
153 ± 72 |
271 ± 135 |
303 ± 134 |
||
|
Methanol |
61 ± 39 |
248 ± 33 |
159 ± 59 |
||
|
Lead Acetate |
76 ± 31 |
112 ± 58 |
79 ± 25 |
||
|
Reaction to light, duration, ms |
Control |
13 ± 3 |
9 ± 2 |
15 ± 2 |
|
|
Phenylcarbamate |
14 ± 2 |
9 ± 4 |
16 ± 2 |
||
|
Methanol |
7 ± 1* |
9 ± 1 |
21 ± 2* |
||
|
Lead Acetate |
20 ± 5 |
9 ± 4 |
31 ± 2● |
||
|
Response to an electrical stimulus, amplitude, units |
Control |
224 ± 46 |
293 ± 111 |
618 ± 80 |
|
|
Phenylcarbamate |
283 ± 67 |
358 ± 78 |
537 ± 145 |
||
|
Methanol |
46 ± 15♦ |
337 ± 60 |
315 ± 63♦ |
||
|
Lead Acetate |
202 ± 70 |
360 ± 140 |
221 ± 74♦ |
||
Table prepared by the authors based on their own data
Notes: the data is presented as the mean value and the standard error of the mean (M ± m); the indicator “water consumption” is presented as the mean group value; “–” — not investigated; statistically significant differences with the control animal group: * — p < 0.05; ♦ — p < 0.01; ● — p < 0.001.
Table 1 indicates that early stages after acute intoxication (seven days) were associated with a statistically significant increase in water consumption in animals treated with phenylcarbamate and methanol. Additional water intake is explained by the need to reduce the concentration of the toxicant and accelerate its excretion from the body.
In the methanol exposure group, male rats demonstrated a decrease in search and research activity of 2.8 ± 0.7 units versus 9.2 ± 1.7 units in control animals, which is a manifestation of the direct toxic effect of the substance. The animals also showed disorders in the reproduction of conditioned reflexes in the CPAR test, a post-stress effect in animals from the phenylcarbamate group of 33 ± 13 s versus 4 ± 3 s in the control group, a shortening of the reaction time to a flash of light of 7 ± 1 ms versus 13 ± 3 ms in the control group. The group of animals exposed to methanol demonstrated a decrease in the amplitude of the reaction in response to an electrical stimulus of 46 ± 15 units, compared with animals from the control group, 224 ± 46 units. These signs correspond to a prolonged period of acute intoxication pattern.
In the following periods, the severity of the above changes was mostly leveled. At the end point of the study (day 28), the animals from the PC group retained increased water consumption and impaired conditioned reflex reproduction, which had been noted in the early stages after intoxication.
In animals exposed to methanol, the initial increase in water consumption was replaced by its decrease on day 21. Along with this, the early increase in the duration of the reaction to a flash of light and a decrease in the amplitude of the response to an electrical stimulus were leveled by the end of the experiment.
In the group of animals who survived lead acetate poisoning, a decrease in water consumption was noted throughout the observation period with its increase by day 28. These animals also demonstrated disorders of conditioned reflexes reproduction, reactions to light (31 ± 2 ms versus 15 ± 2 ms in the control) and sound, with these indicators being more significant than those in the methanol-exposed group. In addition, these animals were unable to perform a treadmill running test; in the open field test, they showed some fussiness, accompanied by an increased search and research activity.
An analysis of the detected dynamics of toxic effects and neurophysiological indicators revealed signs that are sensitive to the formation of long-term consequences of acute poisoning. These include impaired ingestive behavior and its endocrine regulation (decreased water consumption), impaired conditioned reflex reproduction, increased sensitivity to stress (time spent in a dark chamber in the CPAR test), inability to perform the treadmill running test at a high speed of the tape, inhibition in the polysynaptic pathways (high reaction time light in the TSE Startle Response System test), and insufficiency (exhaustion) of activation effects (low amplitude of the response to an electrical stimulus in the TSE Starter Response System test). Altogether, these signs made it possible to obtain an integral quantitative value of the level of formation of long-term consequences based on a point-based assessment system.
Individual variations in the studied indicators of brain metabolism were noted, with the corresponding data presented in Table 2.
Table 2. Indicators of brain metabolism in survived animals on day 28 after acute poisoning with neurotoxicants
|
Indicator, unit |
Control (n = 8) |
Phenylcarbamate (n = 9) |
Methanol (n = 10) |
Lead Acetate (n = 5) |
|
NSE, pg/mL |
0.71 ± 0.11 |
0.65 ± 0.07 |
0.99 ± 0.17 |
1.38 ± 0.39* |
|
BDNF, ng/mL |
1.22 ± 0.12 |
1.15 ± 0.04 |
1.30 ± 0.09 |
1.73 ± 0.11* |
|
MBP, ng/mL |
0.06 ± 0.01 |
0.06 ± 0.01 |
0.06 ± 0.01 |
0.05 ± 0.01 |
|
S100, ng/mL |
0.120 ± 0.011 |
0.084 ± 0.007* |
0.087 ± 0.012 |
0.064 ± 0.006* |
|
GSH, mmol/L |
2.00 ± 0.05 |
1.95 ± 0.04 |
1.91 ± 0.04 |
1.94 ± 0.05 |
|
MDA, mmol/L |
19.1 ± 1.7 |
19.2 ± 0.9 |
22.8 ± 2.3 |
18.9 ± 1.0 |
|
DC, mmol/L |
65.3 ± 1.6 |
98.7 ± 36.0 |
126.7 ± 52.9 |
197.2 ± 80.6 |
|
GST, U/g of protein |
62.7 ± 1.2 |
57.8 ± 3.5 |
75.2 ± 6.0 |
56.2 ± 1.2 |
|
GP, U/g of protein |
1.45 ± 0.03 |
1.42 ± 0.08 |
1.76 ± 0.16 |
1.40 ± 0.04 |
|
G6PDH, U/g of protein |
35.8 ± 2.3 |
38.2 ± 3.2 |
49.2 ± 5.0* |
42.4 ± 2.3 |
|
SOD, U/g of protein |
21.5 ± 5.3 |
24.2 ± 5.7 |
50.0 ± 5.4* |
20.5 ± 5.4 |
Table prepared by the authors using their own data
Note: the data are presented as the mean value and the standard error of the mean (M ± m); statistically significant differences with the group of intact animals: * — p < 0.05.
An analysis of the data presented in Table 2 shows a moderate decrease in S100 protein by 30% (p < 0.05) in survivors on day 28 after poisoning (the period of formation of long-term consequences of acute poisoning) under the influence of acute phenylcarbamate intoxication.
The group of animals exposed to methanol poisoning showed a statistically significant increase in the activity of SOD by 90% and G-6-FDG by 37%, as well as a statistical tendency to decrease the activity of S100 protein by 27% with an increase in the activity of antioxidant defense enzymes (glutathione transferase and glutathione peroxidase by 20–21%). This observation may be indicative of compensatory processes in brain tissue in the post-intoxication period.
On day 28 after acute intoxication with lead acetate, survived animals demonstrated a statistical tendency to increase the marker of neuronal damage NSE by 94% and a threefold increase in the concentration of LPO final products — DCs — with a decrease in glutathione transferase activity by 10%, reflecting the neurotoxicity of this compound. However, in comparision with other toxicants, lead acetate exposure resulted in a more pronounced, statistically significant decrease in the activity of S100 protein by 46% and MBP by 23%, an increase in the activity of the brain neurotrophic factor BDNF by 42%, as well as a moderate increase in the activity of G-6-FDG by 18%. These indicators manifest a high activity of compensatory and adaptive biochemical mechanisms during this period.
It should be noted that neurospecific proteins detected in blood serum 30 days after acute poisoning proved to be uninformative for detecting and assessing the severity of long-term health effects of intoxication. Only for lead acetate, metabolic signs of neuronal damage and, probably, increased permeability of the blood–brain barrier were found. It is important that lead acetate is characterized by the activation of LPO processes in the setting of a decrease in the activity of the enzymatic link of the antioxidant system. These findings are likely to be correlated with the ability known for heavy metals to inhibit the enzymatic activity of a wide range of enzymes due to binding to thiol groups in the active site.
Figure 2 demonstrates slice micrographs of the rat brain temporal cortex to assess the effect of toxicants on the processes of neuronal apoptosis.
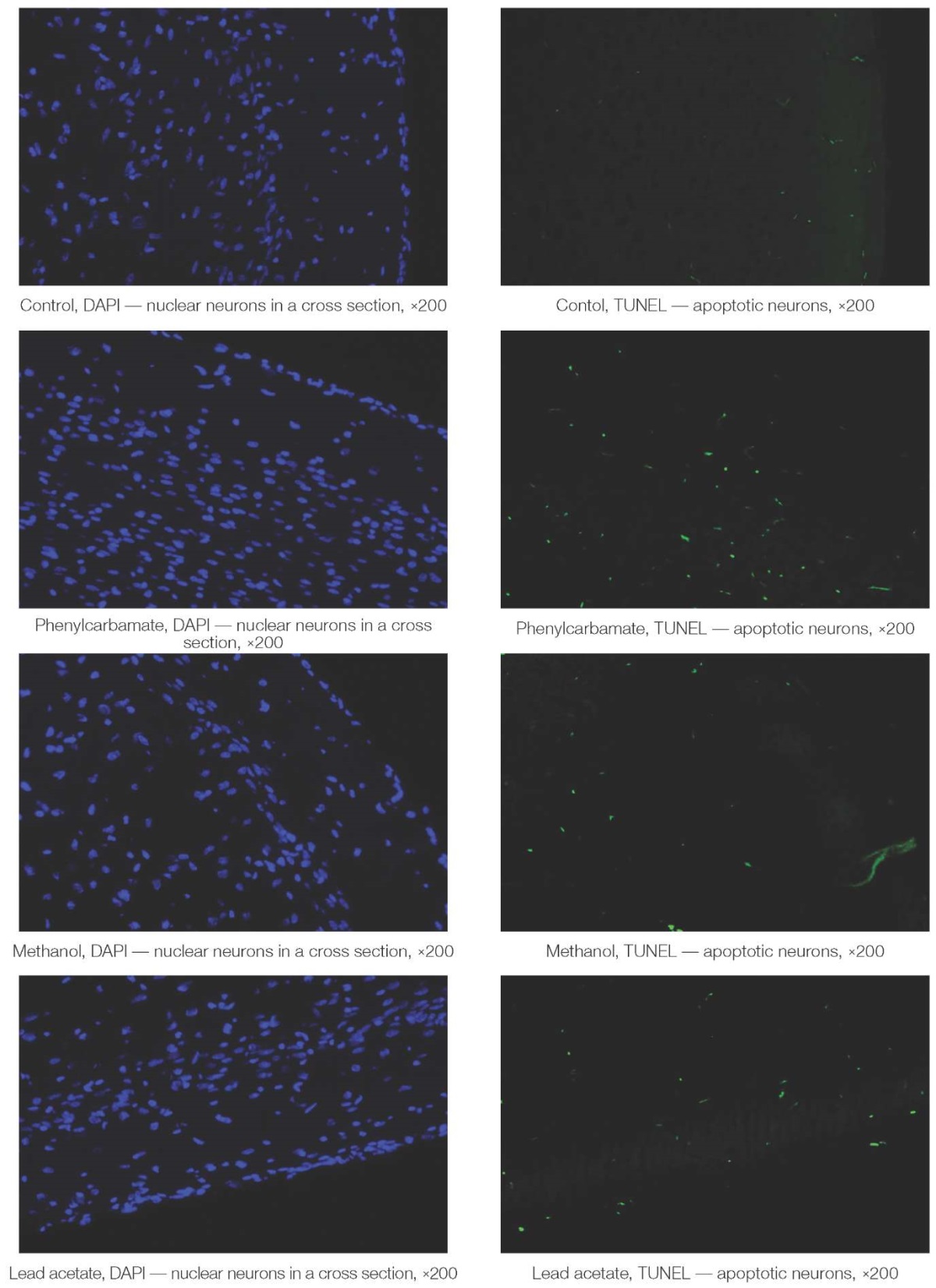
Figure prepared by the authors using their own data
Fig. 2. Slice micrographs of the rat brain temporal cortex
Under almost the same number of blue-colored nuclear neurons on a slice of the cerebral cortex (left photos), the number of apoptotic TUNEL-positive nerve cells in the survived animals significantly increased under the influence of toxicants (green glow, right row of photos, Fig. 2).
Figure 3 presents the results of assessing the activity of apoptotic processes.
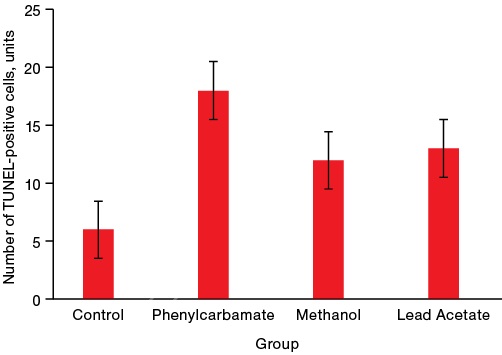
Figure prepared by the authors using their own data
Fig. 3. The activity of apoptotic processes in the cells of the rat brain temporal cortex on day 28 after acute poisoning with neurotoxicants
Note: * p < 0.05 — statistically significant differences from the control group
The data presented in Fig. 3 shows that all the studied toxicants, regardless of the mechanisms of their toxic effect, increase the number of neurons in the state of apoptosis by 3–4 times, thereby being the inducers of this pathological process.
A variance factor analysis showed that the severity of neuron apoptosis in the rat cerebral cortex is closely related to the formation of long-term consequences of severe acute poisoning with neurotropic toxicants. This indicator is associated with more than 20% of the total variability in the assessment of their formation (R2 = 0.47, p = 0.034) in survivor animals. In this regard, the subsequent analysis was focused on assessing the significance of the influence of the studied metabolic factors on the activity level of neuronal apoptosis processes.
It follows from Fig. 3 that, on day 28, a pronounced sharp activation of apoptotic processes was observed in the brain cells of the survived animals, which is more characteristic of phenylcarbamate. The variance factor analysis showed that the controlled factor of intoxication fact determines 64% of the total variability (p = 5×10-8) of the apoptosis index. The type of neurotoxicant is even more important in activating apoptosis (72% of the total variation of the trait, p = 2×10-6), while the coefficient of determination for phenylcarbamate equaled D = 0.97 (p = 5×10-13), for methanol D = 0.86 (p = 4×10-8), for lead acetate D = 0.80 (p = 4×10-5).
In order to assess the level of apoptosis activation during the formation of long-term health consequences of acute poisoning, a frequency analysis and S-scaling were performed. To that end, the inflection points on the curves of the statistical distribution of the number of TUNEL-positive cells in slices were determined (Fig. 4), which are the boundaries of the levels of apoptosis activation. If the indicator under study can be characterized as close to a normal statistical distribution, such criterion points on the cumulative curve will be 16% (the boundary of low and medium levels), 66% (the boundary of medium and elevated levels), and 84% (the boundary of elevated and high levels). It is noteworthy that apoptosis induction was more pronounced (by four times) when exposed to phenylcarbamate, while the apoptosis-inducing activity of methanol and lead acetate was weaker (by about 30%).
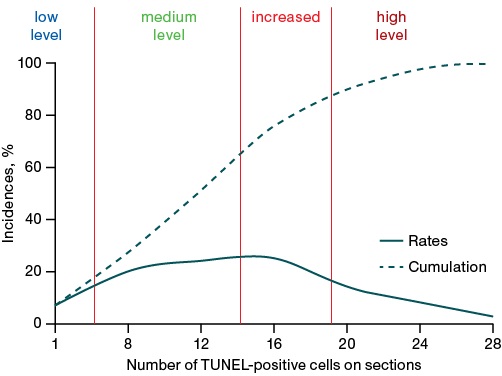
Figure prepared by the authors using their own data
Fig. 4. Frequency distribution curves of the number of TUNEL-positive cells on sections of the rat brain temporal cortex
The boundaries of the ranges (Fig. 4) are as follows: low-level — 6 or fewer TUNEL-positive cells per slice; medium-level — 7–10 cells, elevated — 14–18 cells, high-level — 19 or more cells per slice.
The following structure of apoptosis activation levels was revealed in the experimental groups (Table 3).
Table 3. Distribution of animals by levels of apoptosis activation under the influence of neurotoxicants
|
Group |
Level of apoptosis activation |
|||
|
low |
moderate |
increased |
high |
|
|
Control |
88 |
12 |
– |
– |
|
Phenylcarbamate (1.6 mg/kg bw) |
– |
– |
33 |
67 |
|
Methanol (11.5 g/kg bw) |
– |
100 |
– |
– |
|
Lead Acetate (300 mg/kg bw) |
– |
80 |
– |
20 |
Table prepared by the authors using their own data
Note: the data is presented in the form of % incidence in the group.
Table 3 shows that phenylcarbamate is a strong apoptosis inducer, causing predominantly a high level of its activation, while methanol and lead acetate cause moderate activation of programmed cell death of neurons.
The conducted analysis of the role of individual components of the brain metabolic systems in the activation of apoptotic processes under the action of neurotoxicants found that the studied metabolic parameters do not play a significant role in the process of brain cell apoptosis at the stage of formation of the long-term consequences of severe acute poisoning with neurotropic toxicants. Thus, their coefficients of determination ranged from 0.02 to 0.11, not being statistically significant. For neurospecific proteins, this may probably be due to both the features of toxic brain damage at the tissue level and the timing after acute intoxication, which triggered the activation of apoptosis. Thus, NSE is known to reach a maximum in cerebrospinal fluid one day after an ischemic or hemorrhagic stroke, decreasing by days 4–5 and being more specific to focal brain damage than diffuse [12–15]. S100 protein also shows typical periods for increasing its concentration, i.e., days 2–7. MBP is a marker of axonal damage and demyelination, which may not have formed by the time of 30 days from the moment of acute intoxication. BDNF is more overexpressed with a lack of neuronal plasticity, and the weak response of this neurotrophin to brain tissue damage is clinically more significant [17–18].
In the group of intact animals, the apoptosis level had no significant correlations with either neurospecific proteins or LPO indicators and the antioxidant system (AOS), being a spontaneous process. In the group of animals exposed to phenylcarbamate intoxication, the apoptosis level was modestly affected by a low level of reduced glutathione (r = –0.53). After methanol intoxication, a weak correlation between apoptosis and the content of the main protein myelin (r = +0.47) was revealed, most likely reflecting the presence of a common inducing factor for them. After intoxication with lead acetate, the activity of neuronal apoptotic processes was associated with a low activity of glutathione transferase (r = –0.66) and SOD (r = –0.47). Therefore, the use of activators of these enzymes can be useful in the set of measures for preventing the long-term consequences of poisoning with organic lead derivatives.
NSE in the group of intact animals showed moderate negative associations with the activity of glutathione-dependent AOS enzymes — GST (r = –0.63) and GP (r = –0.57), i.e., an increase in the content of this marker protein may reflect the weakness of the AOS enzymatic link. At the same time, a positive correlation was found with the level of reduced glutathione (r = +0.75). Taken together, these connections may indicate a link between the damage to the bodies of neurons and the disorder of the ability to utilize reduced glutathione in antioxidant defense reactions, since the latter results in a simultaneous increase in its level and a decrease in the activity of utilization enzymes. This pattern is also observed in phenylcarbamate poisoning; however, there is an additional decrease in glucose-6-phosphate dehydrogenase activity (r = –0.67) and a positive correlation with S100 protein (r = +0.70). Following methanol intoxication, accumulation of utilized reduced glutathione (r = +0.55) is also noted; however, this is combined with lower BDNF values (r = –0.50). A peculiar pattern of NSE relationships is noted after lead acetate intoxication. An alternative activation of the expression of either NSE or S100 protein was revealed (with the correlation coefficient between them of r = –0.81). Moreover, the low activity of AOS shifts the system toward increasing the level of S100 protein. In lead intoxication, a pronounced positive correlation (almost linear) was found between the level of NSE and the activity of SOD (r = +0.94), which implies a single response to lead poisoning. After phenylcarbamate poisoning, this marker negatively correlates with the activity of glutathione-dependent AOS enzymes. In case of methanol poisoning, an increase in the amount of this protein in brain tissues is associated with the accumulation of MDA (r = +0.61) and a compensatory substrate increase in SOD activity (r = +0.57). A similar correlation between MBR and SOD was also found after lead acetate poisoning (r = +0.53).
In phenylcarbamate poisoning, the BDNF level correlated with diene conjugates (r = +0.61). After methanol poisoning, it was correlated with a deficiency of reduced glutathione (r = –0.60). After lead poisoning, the neuroprotective effects of BDNF were recorded, including a decrease in axonal damage by the S100 protein marker (r = –0.66) and an improvement in antioxidant protection (a decrease in diene conjugates (r = –0.62), an increase in the activity of glutathione transferase (r = +0.68), glutathione peroxidase (r = +0.61) and SOD (r = +0.49).
Protein S100 in the group of intact animals and in methanol poisoning showed a moderate correlation with SOD (r = +0.62). After phenylcarbamate poisoning, it correlates with the level of NSE (r = +0.70), which may reflect the process of parallel damage to both neuron bodies and axons. After poisoning with lead acetate, the level of S100 protein increases with insufficiency of the antioxidant system, i.e., low levels of reduced glutathione (r = –0.51), GST (r = –0.61), and, in particular, GP (r = –0.84) and SOD (r = –0.75).
With regard to LPO and AOS indicators, a dense cluster of positively correlating activity indicators of glutathione-dependent enzymes (GST and GP) and glucose-6-phosphate dehydrogenase was isolated in the brain tissues of intact animals (correlation coefficients in the range from +0.70 to +0.98). In case of poisoning with phenylcarbamate and methanol, SOD joins this cluster, the density of correlations decreases slightly. At the same time, after poisoning with lead acetate, this cluster disintegrates, glutathione peroxidase becomes the key antioxidant enzyme, and SOD is associated with a moderate correlation with glucose-6-phosphate dehydrogenase.
CONCLUSION
The conducted research showed that the common effects shared by the analyzed toxicants include an increase in the number of neurons dying by apoptosis (most pronounced with phenylcarbamate intoxication) and a decrease in the blood serum level of S100 protein (most pronounced with lead acetate poisoning). Moderate activation of antioxidant protection enzymes was specific for the long-term effects of methanol poisoning, likely as a compensatory reaction against the activation of LPO processes. The long-term effects of lead acetate poisoning in survivor animals were manifested by increased serum levels of NSE and BDNF, decreased protein S100 and MBP, increased levels of diene conjugates with decreased GST activity, and moderate activation of glucose-6-phosphate dehydrogenase. The latter is a key enzyme in the pentose phosphate pathway for the formation of reduced forms of coenzymes for the oxidation of energy metabolism substrates in brain tissues.
It was established that phenylcarbamate exhibits the properties of a strong inducer of apoptosis of cerebral cortex cells, while methanol and lead acetate are inducers with moderate activity.
It should be noted that the calculated coefficients of determination exhibit low or moderate values (for glutathione transferase and glutathione peroxidase). This suggests that neurospecific proteins, lipid peroxidation and antioxidant protection processes do not significantly affect the processes of neuronal apoptosis in survived animals at the stage of formation of long-term health consequences of severe acute poisoning. In this regard, future research should address the effect of metabolic processes in animal brain tissue on the activity of apoptotic processes and the formation of long-term consequences at an earlier stage after acute intoxication with neurotropic toxicants.
1. FMBA Guidelines 12.08-2021 Clinic, diagnosis and treatment of chronic poisoning (exposure) to neurotoxic substances. 2021. (In Russ.)
2. Badalyan AV., Belova MV., Brusin KM. et al. Medical Toxicology: National guidelines. М.: GEOTAR-Media, 2014. (In Russ.)
References
1. Krasnov VN. Psycho-organic syndrome as a subject of neuropsychiatry. Doktor.Ru. 2011;(4):34–42 (In Russ.). EDN: OYYBSX
2. Brendan L. McNeish, Noah Kolb. Toxic Neuropathies. Continuum (Minneap Minn). 2023;29(5):1444–68. https://doi.org/10.1212/CON.0000000000001343
3. Gaikova ON, Kozlov AA, Katretskaya GG, Melnikova MV, Melekhova AS, Bondarenko AA, et al. Мorphological characteristics of toxic brain damage. Extreme medicine. 2024;26(2):13–9 (In Russ.). https://doi.org/10.47183/mes.2024.025
4. Deev RV, Bilyalov AI, Zhampeisov TM. Modern concepts of cell death. Genes and Cells. 2018; 13(1):6–19 (In Russ.). https://doi.org/10.23868/201805001
5. Kashuro VA. Dynamics of the content of neurotrophic factors of the brain in experimental coma in rats. Kazan Medical Journal. 2013;(94):695–9 (In Russ.). EDN: RSHIDV
6. Kostrova TA. Experimental assessment of changes in neurotrophic and apoptotic factors in the long-term effects of acute severe sodium thiopental poisoning. Toxicological bulletin. 2019;(5):49–53 (In Russ.). https://doi.org/10.36946/0869-7922-2019-5-49-53
7. Luzhnikov EA. The specific features of the development and course of toxicohypoxic encephalopathy in acute poisoning by neurotoxic agents. Anesthesiology and reanimatology. 2005;6:4–8 (In Russ.). EDN: HVKWSV
8. Petrov AN, Voytsekhovich KO, Melekhova AS, Lisitskiy DS, Bel’skaya AV, Mikhaylova MV, Gaykova ON. Problems of diagnostics of neurotoxic disorders — the effects of convulsive substances poisoning. Bulletin of the Russian Military Medical Academy. 2017;3(59):211–7 (In Russ.). EDN: ZOWOBD
9. Bespalov AYa, Prokopenko LI, Gorchakova TL, Kozlov VK, Petrov AN, Zaytseva MA, et al. Hydrochlorides of substituted 2-[(dimethylamino)methyl] aryl dimethyl carbamates with anticholinesterase activity. Patent of the Russian Federation No. 2754133;2021 (In Russ.).
10. Kapanadze GD, Revyakin AO, Shustov EB. Methodology for evaluating the detoxification system of xenobiotics in laboratory animals. Biomedicine. 2017;3:71–81 (In Russ.).EDN: ZHYWKB
11. Kostrova TA, Batotsyrenova EG, Kashuro VA, Dolgo-Saburov VB, Stepanov SV, Zolotoverhaya EA, et al. Evaluation of biochemical parameters in the brain tissues of rats in the long-term period after severe sodium thiopental poisoning. Medicine of Extreme Situations, 2019;21(3):429–35 (In Russ.). EDN: GGIEFQ
12. Maksimova MYu, Ionova VG, Syskina AA, Shabalina AA, Kostyreva MA, Senektutova OA. Neurospecific peptides in the assessment of brain damage in patients with atherothrombotic stroke. Annals of Clinical and Experimental Neurology. 2011;5(3):4–10 (In Russ.). EDN: OOKEFR
13. Wunderlich MT, Lins H, Skalej M, Wallesch C-W, Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin Neurol Neurosurg.2006; 108(6):558–63. https://doi.org/10.1016/j.clineuro.2005.12.006
14. Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. Journal of Neurotrauma. 2007;(24):1793–1801. https://doi.org/10.1089/neu.2007.0316
15. Vlasakova K, Tsuchiya T, Garfinkel IN, et al. Performance of biomarkers NF-L, NSE, Tau and GFAP in blood and cerebrospinal fluid in rat for the detection of nervous system injury. Front Neurosci. 2024;(17):1285359. https://doi.org/10.3389/fnins.2023.1285359
16. Klimenko AL, Deev AI, Baskakov IS, Bogdanova MN, Zabirova AKh, Mazikina AN. Neurospecific proteins of s100 family and metal-ligand homeostasis in etiopathogenesis of ischemic stroke: a literature review. Trace elements in medicine. 2019;20(1):3–13(In Russ.). https://doi.org/10.19112/2413-6174-2019-20-4-3-13
17. Levchuk LA, Bokhan NA, Ivanova SA. Neurospecific Proteins as Transdiagnostic Markers of Affective Disorders. Neurochemistry. 2023;40(1):30–4 (In Russ.). https://doi.org/10.31857/S1027813323010119
18. Lima Giacobbo B, Doorduin J, Klein HC, Dierckx RAJO, Bromberg E, de Vries EFJ. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol Neurobiol. 2019;56(5):3295–3312. https://doi.org/10.1007/s12035-018-1283-6
About the Authors
E. B. ShustovRussian Federation
Evgeny B. Shustov
St. Petersburg
M. V. Melnikova
Russian Federation
Margarita V. Melnikova
St. Petersburg
K. V. Masterova
Russian Federation
Kristina V. Masterova
St. Petersburg
V. F. Ostrov
Russian Federation
Vladimir F. Ostrov
St. Petersburg
E. A. Zolotoverkhaja
Russian Federation
Ekaterina A. Zolotoverkhaja
St. Petersburg
L. G. Kubarskaya
Russian Federation
Larisa G. Kubarskaya
St. Petersburg
K. O. Tanayants
Russian Federation
Ksenia O. Tanayants
St. Petersburg
A. A. Kozlov
Russian Federation
Alexander A. Kozlov
St. Petersburg
Yu. O. Sokolova
Russian Federation
Yulia O. Sokolova
St. Petersburg
P. K. Potapov
Russian Federation
Petr K. Potapov
St. Petersburg
Supplementary files
Review
For citations:
Shustov E.B., Melnikova M.V., Masterova K.V., Ostrov V.F., Zolotoverkhaja E.A., Kubarskaya L.G., Tanayants K.O., Kozlov A.A., Sokolova Yu.O., Potapov P.K. Analysis of factors influencing apoptotic processes during formation of long-term health effects of severe acute poisoning with neurotropic toxicants. Extreme Medicine. 2025;27(1):5-14. https://doi.org/10.47183/mes.2024-247

















