Scroll to:
Oxidative stress signs in blood proteome analysis of female volunteers in five-day dry immersion test
https://doi.org/10.47183/mes.2025-251
Abstract
Introduction. Dry immersion is a model for reproducing the physiological effects of weightlessness. Such tests allow assessment of changes in the functions of the cardiovascular, musculoskeletal, and other body systems. Mass spectrometry instruments can be used for blood proteome analysis in order to establish mechanisms of physiological adaptation to spaceflight factors simulated in dry immersion tests.
Objective. To clarify molecular participants in the acute period of adaptation of physiological systems to the conditions of simulated microgravity according to proteome analysis of dry blood spots of participants in a five-day dry immersion test.
Materials and methods. The study involved eight healthy female volunteers (average age 30 ± 4.8 years). Blood proteins were analyzed using the method of dried blood spots; capillary blood dried on a special paper filter (Perkin Elmer) was used. The enzyme cleavage of proteins was performed using trypsin (Thermo Scientific, USA). Mixtures of tryptic peptides were analyzed by liquid chromatography-mass spectrometry (LC–MS) on a Dionex Ultimate 3000 nano-HPLC combined with a TimsTOF Pro mass spectrometer. Mass spectrometric analysis was performed using the method of parallel accumulation with
sequential fragmentation (PASEF). The obtained LC–MS/MS data were semi-quantitatively analyzed using DIA-NN 1.8.1. Statistical analysis was performed in the Statistica 12 software package.
Results. The molecular response to immersion conditions was found to lead to increased levels of antioxidant defense proteins, activation of catabolism processes and the pentose phosphate pathway. The levels of negative regulators of endopeptidases and iron homeostasis proteins decreased. The revealed elevated levels of NADPH oxidase activators indicate activation of NADPH oxidase under experimental conditions. These results may indicate the development of oxidative stress during immersion.
Conclusions. The identified molecular participants in the female body’s response to immersion conditions can provide information about the signaling pathways and mechanisms involved in the response to hypokinesia, and in the future will contribute to the development of pharmacological measures to support the health of female astronauts. These results may also be useful for understanding the processes leading to adverse effects in people with low levels of physical activity.
Keywords
For citations:
Kashirina D.N., Pastushkova L.Kh., Brzhozovskiy A.G., Kononikhin A.S., Nikolaev E.N., Larina I.M. Oxidative stress signs in blood proteome analysis of female volunteers in five-day dry immersion test. Extreme Medicine. 2025;27(2):205-212. https://doi.org/10.47183/mes.2025-251
INTRODUCTION
Dry immersion (DI) is an effective ground-based model for assessing the impact of initial stages of space flight on the astronaut health. In comparision with other models, such as head-down bed rest (HDBR), DI shows a higher adequacy due to the possibility of simulating physiological changes similar to those in the initial period of space flight and their dynamic monitoring [1]. DI simulates a lack of support, immobilization, hypokinesia, and centralization of body fluids, i.e., phenomena observed during space flight [2]. This model can be used to evaluate microgravity-induced changes in the functions of the vestibular apparatus, cardiovascular, musculoskeletal, somatosensory, and other body systems.
The biological response of the human body to spaceflight conditions is manifested in pronounced oxidative stress, which is capable of causing damage to all cellular structures, including DNA. Oxidative stress occurs when the production of free radicals exceeds the natural antioxidant capacity of the cell [3]. Exposure to microgravity and cosmic radiation increases the production of reactive oxygen and nitrogen species (ROS and RNS), thus disrupting the functions of the cardiovascular system and bone tissue [4]. An increase in the level of 8-oxoguanosine (a product of DNA oxidation) in the urine of 59 astronauts was also shown [5]. At the physiological level, oxidative stress and redox imbalance contribute to disturbances in the regulation of metabolism of the cardiovascular, immune, and nervous systems associated with space flight [6].
During ground-based experiments, an increase in various parameters was also observed, indicating the development of oxidative stress [7, 8] as a result of either increased generation of ROS or dysfunction of antioxidant defense systems [9]. Thus, an increase in the marker of oxidative DNA damage — 8-OH-deoxyguanosine — was detected in HDBR, accompanied by an increase in the excretion of markers of bone resorption [N-telopeptide type I collagen (NTX), pyridine crosslinking, deoxypyridinoline] [8]. An increase in iron reserves during space flight is also associated with an increase in oxidative DNA damage and bone loss [10]. During experiments with hypokinesia, the growing ROS generation in muscles not only affects bone metabolism but also leads to changes in the activity of antioxidant systems [11].
The action of microgravity and hypokinesia on human physiological systems is actively studied, including using postgenomic methods. Mass spectrometry-based proteome studies aimed at analyzing dynamic changes in blood proteins, tissues, etc., extend the existing understanding of physiological adaptation mechanisms to spaceflight factors simulated in DI tests.
Thus, previous five-day dry immersion studies detected an increase in the level of free bilirubin and myoglobin in the blood serum, thereby indicating increased levels of hemolysis and myolysis. Increased levels of hepcidin, ferritin, and haptoglobin were also shown, which was attributed to increased levels of serum iron [12]. When comparing changes in iron metabolism in males and females who underwent a five-day DI test, an increase in the systemic availability of iron and serum hepcidin levels was revealed, which indicates an incorrect distribution of iron in these conditions, regardless of the gender [13].
At the same time, changes in the level of a number of proteins both after space flights and in ground-based experiments under conditions of 21-day HDBR and DI were revealed. These proteins include A1BG, A2M, SERPINA1, SERPINA3, SERPING1, SERPINC1, HP, CFB, and TF. This observation indicates changes in the processes affected by microgravity, i.e., hemostasis, platelet degranulation, and protein metabolism [14]. A three-day DI involving female volunteers found significant changes in the transcriptomic profile in the human soleus muscle and a decrease in tissue respiration stimulated by adenosine diphosphate (ADP); however, no changes in the content of mitochondrial proteins/respiratory enzymes was observed. This indicates a regulation disorder of cellular respiration processes. Downregulated RNAs were closely related to mitochondrial function, as well as to lipid metabolism, glycolysis, insulin signaling, and various transporters [15]. In that experiment, the proteome analysis of dry blood spots revealed intracellular proteins with an increased level of expression, which are involved in the processes of pentose phosphate shunt (PGM2, TKT, BPGM). At the same time, the level of extracellular proteins decreased (ALBU, APOA4, AGT, LUM, HPX, SERPINA7) [16].
In the present work, we aim to study the molecular markers of the acute period of physiological adaptation of healthy females to the conditions of simulated microgravity in a five-day dry immersion test according to the proteome analysis of extracts of dry blood spots.
MATERIALS AND METHODS
Design of a five-day dry immersion experiment
Dry immersion is a model for ground-based reproduction of the physiological effects of weightlessness. In our research, testing was organized using the facilities of the Institute for Biomedical Problems of the RAS and conducted at the dry immersion bench base. Eight young healthy female volunteers (average age 30±4.8 years) participated in the study. Each study participant voluntarily signed an informed consent after having been explained the potential risks, benefits, and nature of the upcoming study. A board of medical experts confirmed that all the subjects were in good health and had a normal body mass index. During the period of dry immersion, the subjects were not subjected to any additional effects aimed at correcting adaptive changes in physiological systems. At the onset of the experiment, the female volunteers were synchronized according to the phase of the menstrual cycle (follicular phase) in order to avoid differences in hormonal effects on the studied parameters.
Collection of capillary blood specimens
For the blood proteome analysis, capillary blood dried on a special paper filter (Perkin Elmer) was used (dried blood spot, DBS). The advantages of this method lie in its simplicity and low invasiveness, which makes it possible to collect specimens with high accuracy. Capillary blood specimens were taken from volunteers two days before the onset of the experiment (baseline), on days 1, 3, and 5 during the DI experiment, and two days after the completion of immersion (post-DI). The specimens taken prior to immersion served as a control. Capillary blood was taken from the subjects from the terminal phalanx of the ring finger with an automatic scarifier. Blood in the amount of 20 µL was taken using an automatic pipette; a drop of blood was placed on a special filter paper, dried for 2 h, and stored at minus 20°C.
Dry blood spots were excised and placed in microcentrifuge tubes. Proteins were then extracted, reduced, alkylated, and precipitated as described in [16]. The enzyme cleavage of proteins was performed using trypsin (Thermo Scientific, USA). The obtained mixtures of tryptic peptides were analyzed by liquid chromatography–mass spectrometry (LC–MS) on a Dionex Ultimate 3000 nano-HPLC chromatograph (Thermo Fisher Scientific, USA) combined with a TimsTOF Pro mass spectrometer (Bruker Daltonics, USA). The peptides were separated by an emission packed column (C18, 25 cm×75 µm×1.6 µm; Ion Optics, Parkville, Australia) at a flow rate of 400 nL/min by gradient elution of 4–90% of phase B for 40 min. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in acetonitrile.
Mass spectrometric analysis was performed using the method of parallel accumulation with sequential fragmentation (PASEF). The electrospray ionization (ESI) source operated at a capillary voltage of 1500 V, an end plate offset of 500 V at a temperature of 180°C. The measurements were carried out in the m/z range 100–1700 Th. The ion mobility ranged 0.60–1.60 V s/cm2. The total cycle time was 1.88 s, and the number of PASEO MS/MS scans was set to 10.
The obtained LC–MS/MS data were semi-quantitatively analyzed using DIA-NN 1.8.1. The specified restrictive parameters were as follows: the mass accuracy of the fragments was 1.5e-05 (MS2) and 2e-05 (MS1); the enzyme was trypsin; the maximum number of missing bonds was 3; the fixed modification was carbamidomethyl (C). The threshold for the frequency of false detections (FDR) was set at 0.01. A semi-quantitative analysis was performed using normalized peak intensities in the MS spectra, which reflects the relative levels of proteins in the samples.
Statistical analysis was performed in the Statistica 12 software using the nonparametric Mann–Whitney test (p-value < 0.05). The biological processes, in which the identified proteins are involved, were determined using the STRING web resource1.
RESULTS
As a result of the proteome analysis of DBS samples of female volunteers, 829 different proteins were identified, with about 700 proteins being detected in each sample. When comparing the protein levels at each point of the experiment on day 1, 3, and 5 of DI and post-DI (on the 2nd day after the end) compared to baseline (2 days before the experiment), about 214 proteins were identified. The relative levels of such proteins significantly changed during different periods of the experiment. Upon lengthening of the experiment period, statistically significant differences in the number of proteins responsible for various pathophysiological processes in the body is observed.
On day 1 of immersion, the female participants showed an increase in the levels of four proteins and a decrease in the level of one protein compared to the baseline levels. On day 3, the levels of 18 proteins increased and the levels of 28 proteins decreased compared to the baseline. On day 5, the levels of 43 proteins increased, and 32 proteins decreased. At the same time, an unexpected result was variations in the protein level during the recovery period. Thus, 139 proteins underwent variation, out of which 59 and 80 proteins showed elevated and reduced levels compared to the baseline, respectively.
For all proteins, whose relative levels increased during immersion, nine biological processes were identified using the Gene Ontology (GO) database (Fig. 1A). The most reliable process was the detoxification of cellular oxidants, which included proteins of the CAT, TXNDC17, PRDX1, TXN, TXNRD1, HBB, and HBD genes. Some of these proteins were involved in the process of hydrogen peroxide catabolism (CAT, PRDX1, HB, HBD). Other processes to note were catabolism and biosynthesis of glyceraldehyde-3-phosphate, which is a key intermediate of hexose metabolism in such biochemical processes as glycolysis, gluconeogenesis, pentose phosphate shunt, etc.
Among the biological processes involving proteins with reduced levels during DI (HRG, F12, PEBP1, SERPINA10, AMBP, CLEC3B, USP9X, FN1, SERPINC1, GSN, PSMC2, SERPINA4), only one was identified — regulation of proteolysis (Fig. 1B). Among these proteins involved in the regulation of proteolysis, half of the proteins were protease inhibitors (histidine-rich glycoprotein (HRG); hippocampal cholinergic neurostimulating peptide (PEBP1); protein Z-dependent protease inhibitor (SERPINA10); inter-alpha-trypsin light chain inhibitor (AMBP); antithrombin-III (SERPINC1); callistatin (SERPINA4). A decrease in protease inhibitors is likely to enhance proteolysis under immersion conditions.
The biological process of cellular oxidants detoxification, which is part of antioxidant protection, demonstrated the most statistically significant change. This change was manifested in an increase in protein levels involved in this process. Table 1 lists the antioxidant protection proteins, the levels of which varied throughout the dry immersion period.
Figure 2 presents a scheme of involvement of antioxidant protection proteins in response to immersion conditions. On day 3 of DI, protein 17 containing the thioredoxin domain (TXNDC17), peroxiredoxin-1 (PRDX1), catalase (CAT), and the glutamate-cysteine ligase modifier (GCLM) subunits showed an increase. These subunits exhibit peroxidase activity and promote the removal of cellular hydrogen peroxide, an active form of oxygen capable of damaging cellular components. On day 5, thioredoxin (TRX) and thioredoxin reductase 1 (TXNRD1) were involved in the response. Thioredoxin reduction is carried out by thioredoxin reductase, which uses a single NADPH molecule for this purpose. The levels of beta and delta subunits of hemoglobin (HB, HBD) also demonstrated a growing trend during this period.
The molecular response to the completion of immersion and return to habitual living conditions was also associated with an increase in proteins involved in this process. Thus, The levels of superoxide dismutase (SOD1), beta- and theta-1 subunits of hemoglobin (HB, HBQ1) increased, while those of PRDX1, CAT, TSN, TXNDC17, and TXNRD1 recovered to the pre-experimental values.
In addition, processes associated with antioxidant protection and oxidative stress, such as the pentose phosphate pathway, NADPH oxidase regulation, and iron homeostasis, were also identified. Thus, a significant increase (p-value<0.05) in the proteins of the pentose phosphate pathway (transketolase (TKT) and phosphoglucomutase-2 (PGM2) (Fig. 3A) was noted, presumably required for the formation of NADPH as one of the main components of the antioxidant defense system.
In addition, in our experiment, after the completion of immersion, a significant increase in the level of Ras-related C3 botulinum toxin substrate 1 (RAC1), which is a NADPH oxidase regulatory subunit that stimulates its activity, was detected compared to the baseline level before immersion (Fig. 3B). Another NADPH oxidase regulator is CDC42 [17], which, according to our data, was elevated on day 3 of DI and after the completion of immersion (Fig. 3B). In this chain of interactions, an important link is the adapter protein beta-parvin PARVB (the level was increased on day 3 of immersion). This protein plays a role in the transmission of integrin signals through the integrin-bound kinase ILK (the level was increased only after the completion of immersion) (Fig. 3B) and the activation of the aforementioned GTPases CDC42 and RAC1.
Attention should be paid to decreased levels of iron ion homeostasis proteins (V-type proton ATPase catalytic subunit A (ATP6V1A), BolA-like protein 2 (BOLA2)), which were observed during dry immersion (Fig. 3B). A steady decrease in the levels of these proteins, starting on day 3 of immersion, may indicate changes in iron metabolism caused by experimental conditions.
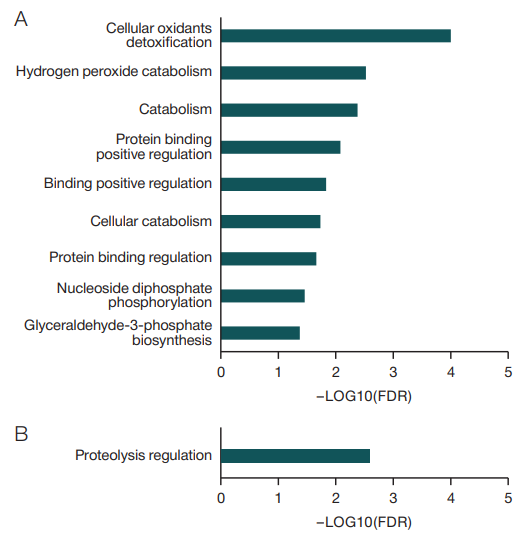
Figure prepared by the authors using their own data
Fig. 1. Statistically significant biological processes involving proteins with altered levels, according to the Gene Ontology database
Note: A — biological processes that include all proteins with significantly elevated levels during immersion; B — biological processes that include all proteins with significantly reduced levels during immersion.
Table 1. Dynamics of changes in levels of antioxidant protection proteins during and after dry immersion as a percentage compared to the baseline levels
|
Proteins |
Genes |
Changes in protein levels compared to baseline, % |
|||
|
DI period, days |
|||||
|
1 |
3 |
5 |
post-DI |
||
|
catalase |
CAT |
109.7 |
119.7* |
125.5* |
114.3 |
|
peroxiredoxin 1 |
PRDX1 |
113.1 |
119.2* |
113.1* |
101.5 |
|
glutamate-cysteine ligase regulatory subunit |
GCLM |
112.9 |
119.1* |
124.0* |
114.0 |
|
glutamate-cysteine ligase catalytic subunit |
GCLC |
108.6 |
112.6 |
111.9 |
125.8* |
|
thioredoxin domain-containing protein 17 |
TXNDC17 |
101.4 |
130.7* |
121.7* |
118.5 |
|
thioredoxin reductase 1, cytoplasmic |
TXNRD1 |
117.8 |
122.4 |
147.2* |
128.6 |
|
thioredoxin |
TXN |
118.1 |
114.7 |
150.6* |
123.8 |
|
hemoglobin subunit delta |
HBD |
118.2 |
109.5 |
126.6* |
108.4 |
|
hemoglobin subunit beta |
HBB |
109.6 |
113.3 |
124.4* |
118.1* |
|
hemoglobin subunit theta 1 |
HBQ1 |
103.5 |
126.7 |
128.4 |
143.7* |
|
superoxide dismutase |
SOD1 |
112.6 |
118.5 |
115.2 |
134.2* |
Table prepared by the authors using their own data
Note: *significant changes compared to the baseline levels (p-value < 0.05).
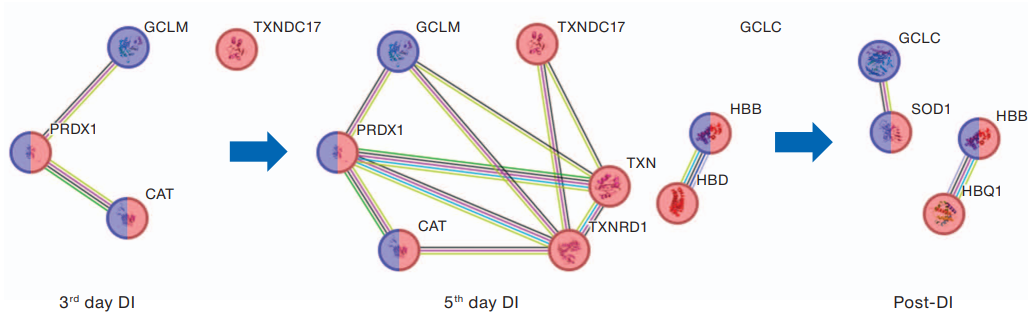
Figure prepared by the authors using their own data
Fig. 2. Relationship of antioxidant protection proteins
Note: red — detoxification proteins of cellular oxidants; blue — proteins of the oxidative stress response; lines of protein–protein interactions: bright green — co-mentioned in Pubmed abstracts; crimson — experimentally determined protein interaction; black — protein co-expression; light blue — the interaction is specified in the verified databases; green — close arrangement of protein genes; blue — joint occurrence of protein genes; lilac — protein homology.
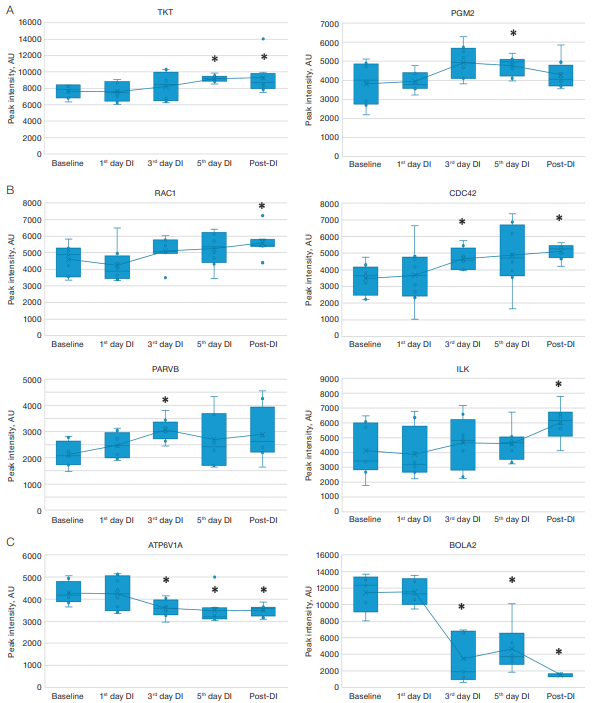
Figure prepared by the authors using their own data
Fig. 3. Changes in protein levels in dry blood spots of participants during a five-day DI test
Note: A — dynamics of changes in the levels of proteins of the pentose phosphate pathway; B — dynamics of changes in NADPH oxidase regulator levels; C — dynamics of changes in iron metabolism protein levels; *statistically significant changes p-value < 0.05; AU — arbitrary unit.
DISCUSSION
The molecular response to five-day immersion conditions is primarily associated with increased levels of antioxidant defense proteins and proteins of the pentose phosphate pathway, which is important for NADPH generation. Interestingly, our three-day immersion experiment conducted earlier also observed an increase in proteins of the pentose phosphate pathway, including the aforementioned PGM2 and TKT [18]. Since the material for the proteomic study was protein extracts of dry blood spots (which, in addition to plasma proteins, also contained cytosol proteins of destroyed blood cells), we believe red blood cells, as the most represented cells of the bloodstream, to make the main contribution to changes in these proteins. In erythrocytes, the pentose phosphate pathway of glucose oxidation provides anabolism processes, being not associated with energy production. In erythrocytes, only NADPH is formed as a product of the pentose phosphate pathway. In this case, pentose is not the final product, but turns into phosphohexose, which closes the cycle or passes into glycolysis, completing the shunt. NADPH is an important component of antioxidant protection. This component is necessary for the regeneration of glutathione, which destroys reactive oxygen species (ROS) together with glutathione peroxidase, as well as for the restoration of thioredoxin with the participation of thioredoxin. Since NADPH is formed in erythrocytes only in reactions of the pentose phosphate shunt, an increase in the concentration of pentose phosphate shunt proteins may be a response to oxidative stress. The results of studies of erythrocyte metabolism conducted before and after space flight showed changes in the metabolic status of cells, expressed in a decrease in the activity of the processes of the regenerative system (a decrease in reduced glutathione) [19], which confirms our assumption.
It has been repeatedly shown that the lack of physical activity increases ROS generation in muscles [11], activates the glutathione system [11][20], and affects the activity of antioxidant systems [11]. Glutathione is a component of one of the main antioxidant systems stimulated both in muscles and at the systemic level (in liver and red blood cells) during activation of oxidative processes [21]. Therefore, it is important for the cell to have sufficient NADPH to restore glutathione and protect against oxidative stress.
We believe that the main cause of oxidative stress observed both during space flight and in model experiments is the effects of physical inactivity and hypokinesia in muscle tissue. There are two main sources of ROS in muscle fibers, i.e., mitochondria, which form ROS during incomplete conjugation of oxidation and phosphorylation [22], and NADPH oxidase-2 (NOX-2), localized in the sarcolemma and inner membranes [23]. It was shown that muscle unloading leads to a decrease in mitochondrial respiration and changes in the operation of NADPH oxidase-2.
NADPH oxidases as the main sources of reactive oxygen species (ROS) in cells continue to attract research interest due to their exceptional function of generating ROS under normal physiological conditions.
Increased levels of NADPH oxidase regulators RAC1_HUMAN, CDC42, as well as proteins signaling these GTPases, PARVB, and ILK during and after immersion confirm the role of NADPH oxidase in ROS generation under hypokinesia conditions and the development of oxidative stress. It is believed that oxidative stress is one of the ways to activate signaling pathways responsible for reducing muscle protein synthesis and activating proteolysis, which subsequently leads to muscle atrophy [24][25].
The modern lifestyle of populations residing in urban agglomerations raises the importance of creating means to prevent the development of oxidative stress and its consequences caused by insufficient physical activity. Indeed, the lack of physical activity leads to increased generation of ROS by vascular cellular components and deteriorates endothelium-dependent vasorelaxation, which contributes to endothelial dysfunction and the development of atherosclerosis. Statins were reported to have a beneficial effect on the cardiovascular system, preventing cardiovascular diseases by blocking RAC1 and NADPH oxidase, thereby reducing ROS generation [26]. It should be noted that countering oxidative stress can also reduce the loss of muscle mass during physical inactivity. Thus, in animal models of hypokinesia, the addition of antioxidants prevented atrophy [27].
The second reason for increased levels of protection proteins from oxidative stress, as well as decreased levels of iron ion homeostasis proteins (ATP6V1A, BOLA2), observed in our dry immersion experiment (Fig. 3B), may be related to increased hemolysis. Activation of hemolysis was detected both in model experiments and during space flight [12][28], although the causes of this phenomenon remain unknown. A thorough assessment of the iron status, as well as hematological reactions, was carried out in a five-day DI experiment involving 20 healthy males [12]. Immersion was found to increase the concentration of iron in the spleen, while iron reserves in the liver were not affected. Sequestration of iron in the spleen was accompanied by an increase in the level of hepcidin in the blood, which suppresses the absorption of iron in the intestine. An increase in the blood level of unconjugated bilirubin, which is normally formed as a result of the breakdown of proteins containing heme (hemoglobin, myoglobin), as well as an increase in myoglobin levels confirm that dry immersion promoted hemolysis and myolysis. These phenomena may explain the simultaneous increase in serum iron levels and transferrin saturation observed in the study [12].
Iron metabolism is strictly controlled at both the systemic and cellular levels. On the one hand, iron is necessary to support many processes in the body; on the other, iron overload can lead to the formation of highly reactive particles during the interaction of labile iron with ROS, which are naturally produced during aerobic respiration in the respiratory chain of mitochondria. These highly reactive particles induce oxidative stress. It is assumed that iron overload contributes to the development of osteoporosis and muscular atrophy by the mechanism described above [29][30].
CONCLUSION
The molecular response to five-day immersion conditions involves changes in the protein composition of extracts of dry blood spots, which leads to increased levels of antioxidant protection proteins, catabolism, protein binding, phosphorylation of nucleoside diphosphates, metabolism of glycolysis intermediates and the pentose phosphate pathway, as well as decreased levels of negative regulators of endopeptidases, iron homeostasis proteins. An interesting finding in our study was the activation of the cellular antioxidant system. Elevated levels of proteins of the pentose phosphate pathway and antioxidant protection may indicate the development of oxidative stress during immersion. Altered iron metabolism may also contribute to ROS generation. The revealed elevated levels of NADPH oxidase activators indicate activation of NADPH oxidase under the conditions of physical inactivity.
The modification of iron metabolism and hemolysis processes, which was observed when simulating the effects of microgravity and hypokinesia, can also contribute to the development of oxidative stress. These observations underscore the importance of iron metabolism assessment in patients with limited mobility. The identified molecular participants in the body’s response to immersion conditions can provide information about signaling pathways and mechanisms involved in the response to hypokinesia or simulated microgravity. The results can be used when developing pharmacological measures to support the health of both astronauts and people with lower levels of physical activity.
1. Search Tool for the Retrieval of Interacting Genes/Proteins — https://string-db.org
References
1. Tomilovskaya E, Shigueva T, Sayenko D, Rukavishnikov I, Kozlovskaya I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 2019;10:284. https://doi.org/10.3389/fphys.2019.00284
2. Navasiolava NM, Custaud M-A, Tomilovskaya ES, Larina IM, Mano T, Gauquelin-Koch G, et al. Long-term dry immersion: review and prospects. Eur. J. Appl. Physiol. 2011;111:1235–60. https://doi.org/10.1007/s00421-010-1750-x
3. Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer and Metastasis Reviews. 2004;23:311–22. https://doi.org/10.1023/B:CANC.0000031769.14728.bc
4. Tahimic CGT, Globus RK. Redox signaling and its impact on skeletal and vascular responses to spaceflight. Int. J. Mol. Sci. 2017;18(10):2153. https://doi.org/10.3390/ijms18102153
5. da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell. 2020;183(5):1185–201.e20. https://doi.org/10.1016/j.cell.2020.11.002
6. Afshinnekoo E, Scott RT, MacKay MJ, Pariset E, Cekanaviciute E, Barker R, et al. Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell. 2020;183(5):1162–84. https://doi.org/10.1016/j.cell.2020.10.050
7. Debevec T, Pialoux V, Ehrstrom S, Ribon A, Eiken O, Mekjavic IB, Millet GP. FemHab: the effects of bed rest and hypoxia on oxidative stress in healthy women. J. Appl. Physiol. 2016;120(8):930–8. https://doi.org/10.1152/japplphysiol.00919.2015
8. Zwart SR, Oliver SA, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat. Space Environ. Med. 2009;80(Suppl 5):A15–22. https://doi.org/10.3357/asem.br07.2009
9. Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009;15(10):468–77. https://doi.org/10.1016/j.molmed.2009.08.004
10. Zwart SR, Morgan JLL, Smith SM. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 2013;98(1):217–23. https://doi.org/10.3945/ajcn.112.056465
11. Agostini F, Dalla Libera L, Rittweger J, Mazzucco S, Jurdana M, Mekjavic IB, et al. Effects of inactivity on human muscle glutathione synthesis by a double-tracer and single-biopsy approach. J. Physiol. 2010;588(Pt 24):5089–104. https://doi.org/10.1113/jphysiol.2010.198283
12. Nay K, Koechlin-Ramonatxo C, Rochdi S, Island ML, Orfila L, Treffel L, et al. Simulated microgravity disturbs iron metabolism and distribution in humans: Lessons from dry immersion, an innovative ground-based human model. FASEB J. 2020;34(11):14920–9. https://doi.org/10.1096/fj.202001199rr
13. Horeau M, Navasiolava N, Van Ombergen A, Custaud MA, Robin A, Ropert M, et al. Dry immersion rapidly disturbs iron metabolism in men and women: results from the VIVALDI studies. NPJ Microgravity. 2024;10(1):68. https://doi.org/10.1038/s41526-024-00399-z
14. Brzhozovskiy AG, Kononikhin AS, Pastushkova LC, Kashirina DN, Indeykina MI, Popov IA, et al. The effects of spaceflight factors on the human plasma proteome, including both real space missions and ground-based experiments. Int. J. Mol. Sci. 2019;20(13):3194. https://doi.org/10.3390/ijms20133194
15. Popov DV, Makhnovskii PA, Zgoda VG, Gazizova GR, Vepkhvadze TF, Lednev EM, et al. Rapid changes in transcriptomic profile and mitochondrial function in human soleus muscle after 3-day dry immersion. J. Appl. Physiol (1985). 2023;134(5):1256–64. https://doi.org/10.1152/japplphysiol.00048.2023
16. Kashirinа D, Brzhozovskiy A, Sun W, Pastushkova L, Popova O, Rusanov V, et al. Proteomic characterization of dry blood spots of healthy women during simulation the microgravity effects using dry immersion. Front. Physiol. 2022;12:75329. https://doi.org/10.3389/fphys.2021.753291
17. Wang X, Ke Z, Chen G, Xu M, Bower KA, Frank JA, et al. Cdc42dependent activation of NADPH oxidase is involved in ethanolinduced neuronal oxidative stress. PLoS One. 2012;7(5):e38075. https://doi.org/10.1371/journal.pone.0038075
18. Kashirina DN, Pastushkova LKh, Brzhozovsky AG, Kononikhin AS, Nikolaev EN, Larina IM. Effects of 3-day immersion exposure on the blood proteome of female volunteers. Aviakosm. Ecol. Med. 2023;57(2):47–56 (In Russ.). https://doi.org/10.21687/0233-528X-2023-57-2-47-56
19. Ivanova SM, Labetskaya OI. Metabolism of erythrocytes. In: Orbital station “Mir”. Vol. 1. Moscow: “Anikom”, 2001;612–5 (In Russ.).
20. Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, et al. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J. Appl. Physiol. (1985). 2009;107(2):549–57. https://doi.org/10.1152/japplphysiol.00280.2009
21. Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8(5):425–36. https://doi.org/10.1016/j.cmet.2008.09.002
22. Hyatt H, Deminice R, Yoshihara T, Powers SK. Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2019;662:49–60. https://doi.org/10.1016/j.abb.2018.11.005
23. Henríquez-Olguín C, Boronat S, Cabello-Verrugio C, Jaimovich E, Hidalgo E, Jensen TE. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid. Redox Signal. 2019;31:1371–410. https://doi.org/10.1089/ars.2018.7678
24. Shenkman BS. How postural muscle senses disuse? Early signs and signals. Int. J. Mol. Sci. 2020;21(14):5037. https://doi.org/10.3390/ijms21145037
25. Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007;102:2389–97. https://doi.org/10.1152/japplphysiol.01202.2006
26. Kwok JM, Ma CC, Ma S. Recent development in the effects of statins on cardiovascular disease through Rac1 and NADPH oxidase. Vascul. Pharmacol. 2013;58(1–2):21–30. https://doi.org/10.1016/j.vph.2012.10.003
27. Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25(10):3646–60. https://doi.org/10.1096/fj.10-177295
28. Trudel G, Shahin N, Ramsay T, Laneuville O, Louati H. Hemolysis contributes to anemia during long-duration space flight. Nat. Med. 2022;28(1):59–62. https://doi.org/10.1038/s41591-021-01637-7
29. Reardon TF, Allen DG. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009;94:720–30. https://doi.org/10.1113/expphysiol.2008.046045
30. Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, et al. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood. 2010;116(14):2582–9. https://doi.org/10.1182/blood-2009-12-260083
About the Authors
D. N. KashirinaRussian Federation
Daria N. Kashirina, Cand. Sci. (Biol.)
Moscow
L. Kh. Pastushkova
Russian Federation
Ludmila Kh. Pastushkova, Dr. Sci. (Biol.)
Moscow
A. G. Brzhozovskiy
Russian Federation
Alexander G. Brzhozovskiy, Cand. Sci. (Biol.)
Moscow
A. S. Kononikhin
Russian Federation
Alexey S. Kononikhin, Cand. Sci. (Phys. and Math.)
Moscow
E. N. Nikolaev
Russian Federation
Evgeniy N. Nikolaev, Dr. Sci. (Phys. and Math.)
Moscow
I. M. Larina
Russian Federation
Irina M. Larina, Dr. Sci. (Med.)
Moscow
Supplementary files
Review
For citations:
Kashirina D.N., Pastushkova L.Kh., Brzhozovskiy A.G., Kononikhin A.S., Nikolaev E.N., Larina I.M. Oxidative stress signs in blood proteome analysis of female volunteers in five-day dry immersion test. Extreme Medicine. 2025;27(2):205-212. https://doi.org/10.47183/mes.2025-251

















