Scroll to:
Regional cutaneous blood flow in healthy subjects under conditions of 21-day head-down bed rest
https://doi.org/10.47183/mes.2025-267
Abstract
Introduction. Research into the microvasculature as an integral component of the cardiovascular system is particularly relevant in space medicine for identifying adaptive changes to weightlessness and developing new diagnostic criteria for assessing the functional state of an astronaut’s body in long-duration space flights.
Objective. To study the process of microcirculation and its regulation in various skin areas in healthy volunteers under conditions of 21-day head-down (–6°) bed rest (HDBR).
Materials and methods. The experiment involved six male volunteers aged 26–34 years. To simulate translocation of liquid media and physical inactivity, the subjects remained in an antiorthostatic position for 21 days. Microcirculation was studied by laser Doppler flowmetry using LAZMA PF portable laser analyzers (SPE “LAZMA” Ltd, Russia). Participant examination was conducted two days prior the onset of the study, on the 3rd, 7th, 15th, 18th, and 20th day of experimental exposure, as well as two days after the completion of HDBR. Statistical data analysis was performed using the Statistica 13.0 software (IBM, USA).
Results. On day 3 of HDBR exposure, a statistically significant decrease in basal perfusion and the amplitude of myogenic oscillations in the skin of the forehead and shin was observed. The analysis of functional tests on the forearm showed a decrease in the respiratory test index by 10.87% throughout the experimental period. On day 3 of hypomobility, a decrease in the venuloarteriolar response by an average of 10.64% and an increase by 91.82% in the capillary blood flow reserve were noted, with the latter persisting throughout the entire exposure.
Conclusions. The effect of HDBR is expressed in a decrease in skin perfusion against the background of increased tone of terminal arterioles and precapillary sphincters in the forehead and lower legs, which may indicate microcirculation shift toward larger vessels. Despite the skin perfusion stability in the forearm area at rest, the conducted functional tests showed the probability of changes in vasomotor function under the action of HDBR.
Keywords
For citations:
Pashkova D.V., Popova J.A., Fedorovich A.A., Shpakov A.V. Regional cutaneous blood flow in healthy subjects under conditions of 21-day head-down bed rest. Extreme Medicine. 2025;27(1):124-130. https://doi.org/10.47183/mes.2025-267
INTRODUCTION
Weightlessness is known to be a significant factor affecting the state of the cardiovascular system (CVS) during space flights (SF). Its effects are mainly caused by blood redistribution toward the cranial direction. In addition, SF conditions trigger such changes in the cardiovascular system as fluid movement from intravascular compartments toward intracellular spaces, a decrease in the total circulating blood volume, myocardial mass loss, decreased vascular resistance, increased venous return, and orthostatic intolerance after returning to gravity [1–2].
The prospects of long-duration space flights, e.g., to the Moon, Mars, etc., with the possibility of landing on their surface poses the challenge of solving arising medical issues without timely support from the Earth. In this connection, assessment of the functional state of the circulatory system at both macro- and micro-levels can provide the necessary information about the overall adaptational capacity of the body and its resilience to external influences. From this standpoint, the microcirculatory bed as the primary element of the cardiovascular system that responds to environmental changes presents particular research interest [3]. The skin is one of the most accessible objects for studying blood microcirculation; however, account should taken of the cutaneous blood flow being directly involved in thermoregulation [4].
According to N. Charkoudian, the skin microcirculation reacts to heat or cold stress [5], while scientific data in available publications may differ due to the variety of methods used to assess the overall thermal effect [6][7]. Thus, according to Fedorovich et al. [6], a 30-day stay in a hermetic object at a temperature of +30–38 °C and a humidity of 30–50% did not result in an increase in tissue perfusion recorded by laser Doppler flowmetry (LDF) on the forearm. This was explained by the specifics of skin angioarchitectonics and the depth of sensing by measuring equipment. Examination of the skin microcirculatory bed by Yuan et al. using the LDF method in an experiment with 180-day isolation in temperature- and humidity-neutral conditions revealed a decrease in the blood flow response to an acetylcholine test, which indicated the manifestation of endothelial dysfunction [8]. The study of skin microhemocirculation in SF conditions was undertaken in two experiments. In the first study [9], the authors evaluated cutaneous blood flow and endothelium-dependent vasodilation at the forearm level using LDF in combination with acetylcholine iontophoresis during a three-week SF at the Tiangong-2 station in two taikonauts. The authors observed a slight decrease in basal perfusion during and directly after SF, as well as signs of endothelial dysfunction. In the second study [10], conducted on board the ISS with the participation of one space tourist and one professional cosmonaut, a decrease in tissue perfusion and an increase in vascular tone were found on days 2 and 3 of SF in the skin of the first toe and the temporal region of the head. In addition, functional differences were found in the indices of skin perfusion of the upper and lower extremities between the examined astronauts, which the authors attributed to the use of a preventive blood redistribution agent in one of the study participants [10].
The complexity of organizing space experiments and the limited sample of the astronauts being examined, given multicomponent SF factors, impede a comprehensive study of the microcirculatory link and its regulation in these conditions. Earth model experiments can be used to evaluate the effect of weightlessness, including inactivity, lifting of the support load, and redistribution of liquid media in the cranial direction. One of such experiments is head-down bed rest (HDBR) used for modeling the redistribution of body fluids toward the cranial direction.
In this article, we report a study undertaken to investigate microcirculation and its regulation in various areas of the skin in healthy male volunteers under conditions of 21-day head-down bed rest.
MATERIALS AND METHODS
The experiment was conducted on six healthy male volunteers (average age 30.3 ± 5.2 years; weight — 72.8 ± 7.7 kg; height — 177.1 ± 6.3 cm) using a hypogravitation bench at the Institute of Biomedical Problems (Russia). The volunteers were placed in an antiorthostatic position with an angle of inclination of the body relative to the horizon of –6° without the use of preventive physical exercises and a moderate restriction of motor activity for 21 days. The subjects were verticalized for short-term hygiene procedures (once daily), as well as for passive orthostatic testing (on days 6, 14, and 19) and a lower body negative pressure (LBNP) on day 19. For medical reasons, one of the subjects was allowed to participate in the experiment with restrictions: provided that stress tests, including orthostatic ones, were excluded. A detailed description of experimental conditions can be found in [11].
The method of laser Doppler flowmetry (LDF) was used to assess the state of the skin microcirculatory bed. This method is based on probing the area under study using laser radiation and analyzing the reflected signal from red blood cells moving in the bloodstream. The exclusion criteria for this study of the skin microcirculatory bed (MCB) using the LDF method were: age over 40 years, the presence of additional exposure factors (breathing with hyperoxic mixtures, etc.), the presence of nevi and tattoos in the area of sensor application in the subjects. All six participants in the experiment were included in the study group according to these criteria.
To register microcirculation parameters using the LDF method, three LAZMA PF portable laser analyzers (SPE “LAZMA” Ltd, Russia) with the wavelength of the probing radiation of 850 nm were used. One sensor was placed on the non-dominant arm in the Zakharin–Ged area: along the median line of the forearm, 3–4 cm above the ulnar and radial styloid. The second sensor was placed on the anterior-inner surface in the lower third of the shin. The third sensor was applied on the central area of the forehead (Fig. 1).
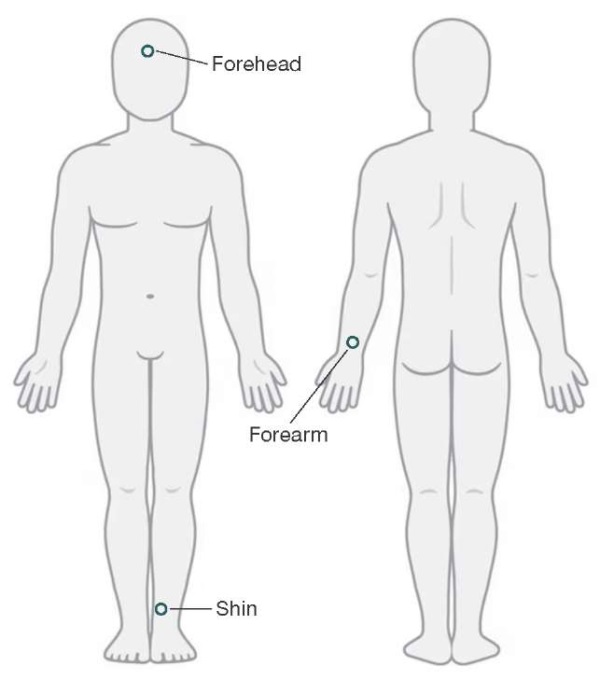
Figure prepared by the authors using data from an open online source
Fig. 1. Placement of portable laser analyzers during the study
The analyzers were fixed using medical tubular elastic bandages. The total examination duration was 45 min. At the first stage, basal perfusion was recorded at rest: the subject was in a relaxed lying position, calmly breathing in a waking state. Following 10 min, functional tests were performed in the forearm area: respiratory and occlusive (venous and arterial) tests. The respiratory test includes a breath-holding maneuver (for 15 sec after rapid deep inhalation through the mouth), allowing assessment of the functional state of sympathetic vasomotor regulation. Venous occlusion was created by increasing pressure in the shoulder cuff to 40 mmHg followed by its maintenance at this level for 2 min. This artificially created an increase in postcapillary pressure, without obstacles to arterial blood flow, to assess the venuloarteriolar constrictor response without involving sympathetic regulation. Arterial occlusion was used to determine the reserve of capillary blood flow, for which pressure was pumped into the shoulder cuff at a level of 30–50 mmHg above the individual systolic pressure and maintained at this level for 3 min, followed by parameter registration in the period of postocclusive hyperemia. The use of these tests allowed us assess the state of the regulation mechanisms of tissue blood flow, as well as the general functional state of the microcirculatory bed in the forearm skin according to the accepted methodology [12][13].
The Lazma specialized software (SPE “LAZMA” Ltd, Russia) was used to calculate the basal perfusion level (M) in perfusion units (PU), as well as the amplitude–frequency spectrum of blood flow oscillations based on wavelet analysis. Thus, the maximum amplitudes of vasomotion were determined in the appropriate frequency ranges, i.e., Ae, An, Am, Av, Ac (endothelial, neurogenic, myogenic, venular (respiratory), and cardiac components of microvascular tone formation, respectively). When performing functional tests, the following indicators were evaluated: BHI as an index of breath holding test, VAR as an venuloarteriolar response, CFR as capillary blood flow reserve (according to the LDF of the forearm skin).
The microcirculatory bed was examined two days prior to the onset of experimental exposure (background study); on days 3, 7, 15, 18, and 20 during HDBR exposure; as well as two days after the completion of exposure (aftereffect period). The studied parameters were recorded prior to and following the HDBR period in the prone position of the subject (15 min after adaptation to the horizontal position). During HDBR exposure, the participants were examined in an antiorthostatic position. The study prior to and following exposure was performed in laboratory conditions with a constant microclimate: ambient temperature — 24.33 ± 2.58 °C; humidity — 57.83 ± 6.97%; atmospheric pressure — 741.17 ± 16.57 mmHg. Under HDBR conditions, the study was conducted in a room with the following environmental parameters: ambient temperature — 23.36 ± 1.34 °C; humidity — 54.57 ± 3.03%; atmospheric pressure — 744.57 ± 17.08 mmHg.
A statistical analysis of the datasets obtained was carried out using the STATISTICA 13.0 statistical software package (IBM, USA) using principal component analysis and the Wilcoxon criterion [14].
RESULTS
When analyzing the data obtained, the study sample of six people was checked for uniformity using principal component analysis for all parameters recorded during the experiment. The results obtained recorded the uniformity of the group, with the values not exceeding two standard deviations.
On day 3 of HDBR stay, the conducted statistical analysis revealed a decrease in the basal perfusion level (p = 0.05, Wilcoxon criterion) by 32.84% in the forehead skin and 22.52% in the shin skin (Fig. 2). In addition, a decrease in this parameter in the shin skin was noted on days 15 and 18 of exposure, amounting to 31.64% and 34.68%, respectively. This may be related to the cyclogram of the experiment, which included an orthostatic test. It should be noted that, by day 3 of hypokinesia, the participant who was not allowed to undergo an orthostatic test and an LBNP test during the HDBR period, showed a 7.02% decrease in blood perfusion in the forehead skin; during the entire HDBR, the downward trend in this parameter persisted and had reached the maximum difference with the background (34.21%) by day 20 of exposure. It could be assumed that changes in skin perfusion might be associated with temperature changes, since the skin is an essential component of the human thermoregulatory system [4].
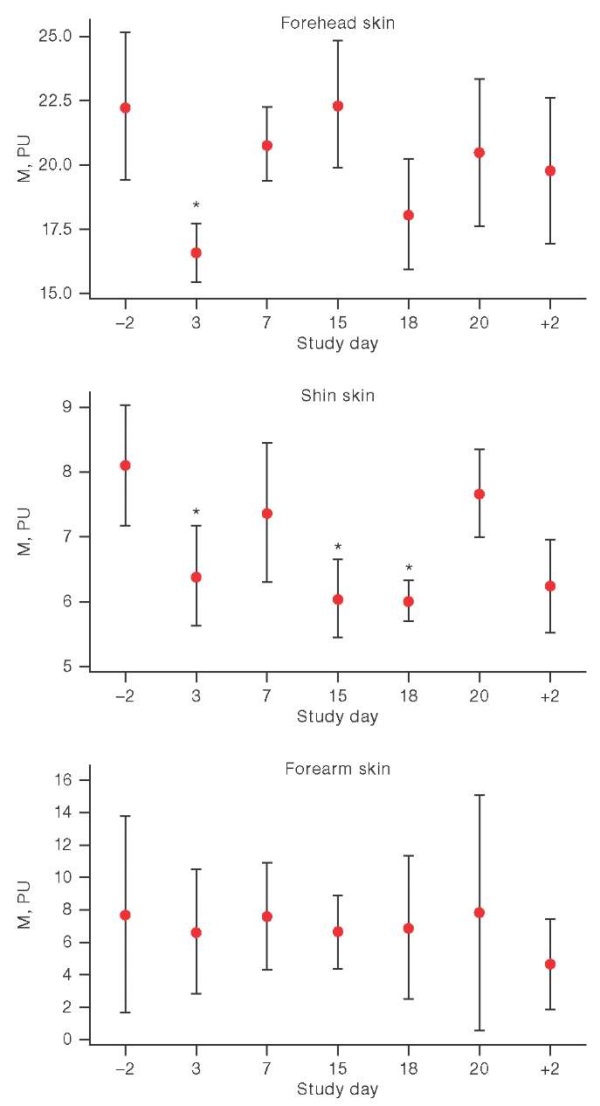
Figure prepared by the authors using their own data
Fig. 2. Basal perfusion changes in HDBR conditions
Note: the graphs show the average values and the interquartile range; on the X-axis: –2 and +2 are the days before and after head-down bed rest; * р < 0.05.
In our study, the ambient temperature during the exposure period was kept at 23.36 ± 1.34 °C, being close to the temperature of the laboratory room in which the studies were conducted before and after HDBR (24.33 ± 2.58 °C). In addition, the temperature of the study area was measured using a sensor built into the LDF analyzer, with the parameter remaining around the same level during the entire experiment. The average temperature was 32.88 ± 1.36 °C in the forehead skin and 32.73 ± 1.38 °C in the shin skin. The humidity level in the rooms during the study practically did not differ between sessions: during the procedure, the windows were closed, and the air conditioning system was turned off before (15 min before the start of the measurement) and during the study. Around 15 min before the onset of the experiment, the participants were not covered with a blanket, the examined skin areas were open (freed from clothing) to adapt to environmental conditions. According to subjective feelings, all volunteers felt comfortable in these conditions. Thus, the effect of fluctuations in environmental parameters on our results was minimal.
A decrease in the amplitude of myogenic oscillations by 51.15% and 41.94% was found in both the forehead and shin skin areas at an early stage of the body’s adaptation to HDBR conditions (day 3). It should be noted that in the head area, the Am value remained below the background (measured 2 days prior to exposure) and decreased relative to the background by 57.03% on day 7, by 47.23% on day 15, and by 47.71% by day 18 (Fig. 3).
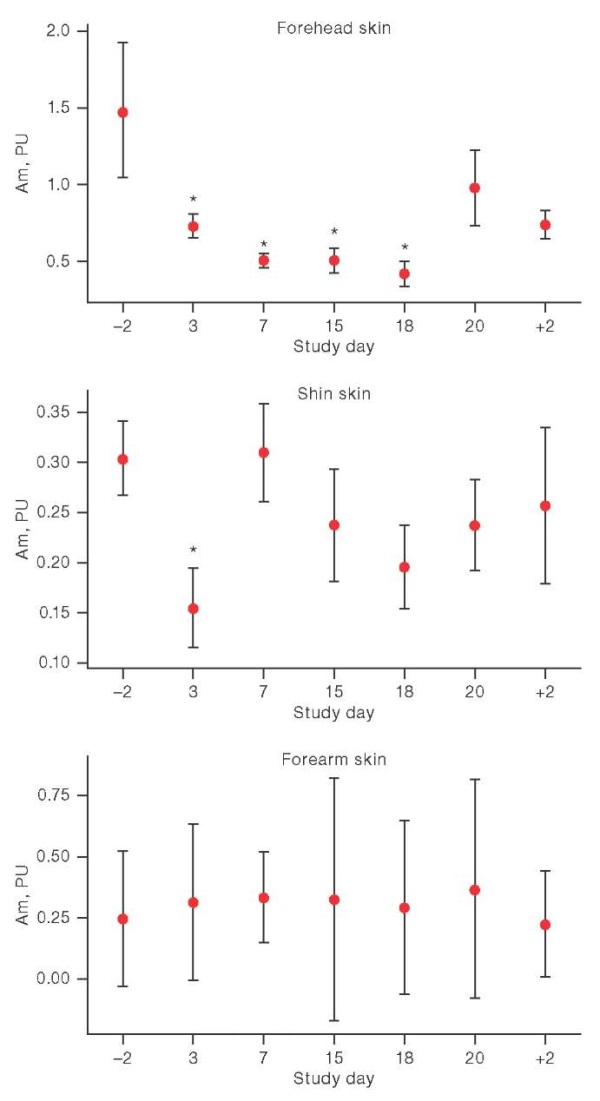
Figure prepared by the authors using their own data
Fig. 3. Changes in the amplitude of myogenic oscillations (Am) under the conditions of head-down bed rest.
Note: the graphs show the average values and the interquartile range; on the X-axis: –2 and +2 are the days before and after head-down bed rest; * р < 0.05.
When analyzing the data obtained during functional tests, changes were found in both the VAR during the breath holding test, and in the VAR and CFR during venous and arterial occlusion, respectively (Fig. 4). However, these changes were multidirectional: when the VAR and BHI decreased, the CFR increased throughout the exposure. Moreover, VAR had a reduced value of relative background exposure on days 3 and 15 of HDBR, BHI — throughout the entire exposure.
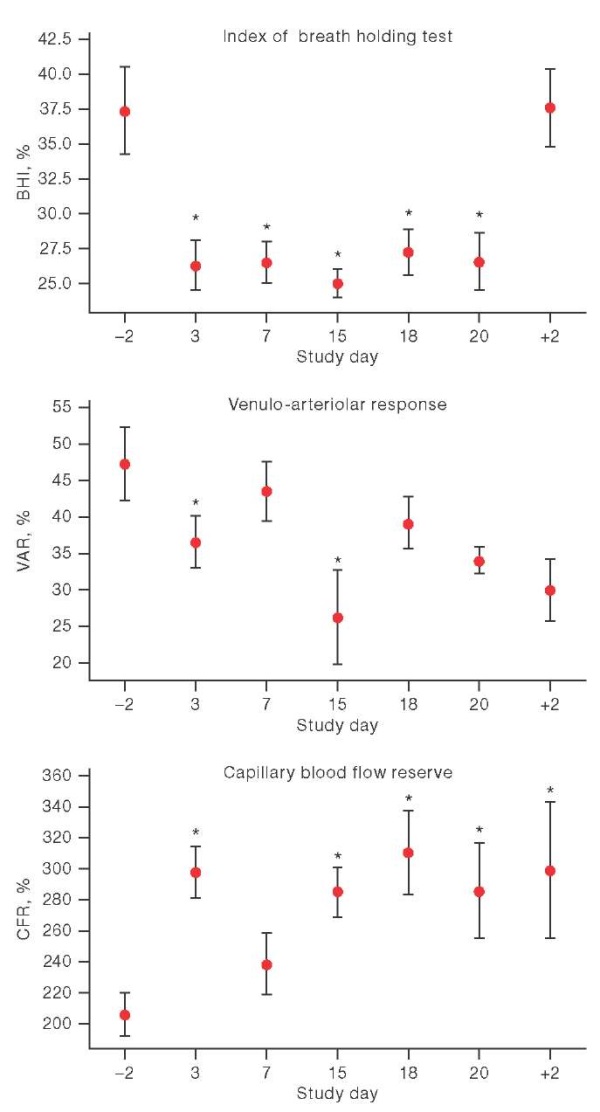
Figure prepared by the authors using their own data
Fig. 4. Parameter dynamics of functional tests in the conditions of head-down bed rest
Note: the graphs show the average values and the interquartile range; on the X-axis: –2 and +2 are the days before and after head-down bed rest; * р < 0.05.
DISCUSSION
It is known that bed rest models primarily simulate such an effect of weightlessness as the redistribution of body fluids toward the cranial direction. In our series of experiments, part of which was the current study, the authors [11] showed that, due to HDBR exposure, the central hemodynamics showed an average daily decrease in heart rate and blood pressure. Previous studies found a decrease in total peripheral resistance [15] and, concerning the peripheral hemodynamics, a decrease in blood flow in the lower extremities [16].
The results obtained in our study indicate that HDBR conditions with an inclination angle of –6° lead to changes in skin microcirculation, which are manifested in a decrease in basal perfusion in the shin and forehead at an early stage of the body’s adaptation (day 3 of exposure). In other words, the changes are unidirectional in both the skin of the lower extremities and the scalp. These data are consistent with the results of a study conducted by Britt et al. [17], who observed a decrease in blood perfusion in the neck area under HDBR conditions.
In an experiment by Kurazumi et al. [18], the subjects briefly placed in an antiorthostatic position demonstrated a decrease in cutaneous blood flow in the cheek area during 10-min antiorthostasis with a body tilt angle of –30°, against which a significant increase in vascular resistance in this area was detected.
In our study, against the background of decreased perfusion, a decrease in the amplitude of myogenic oscillations was noted both in the lower extremities and in the forehead skin. This indicates an increase in vascular tone in the skin microcirculatory bed of these regions. Due to the relatively increased tone of the arterioles, the microvascular lumen narrows, which reduces skin blood flow in the studied areas. It can be assumed that the decrease in the amplitude of myogenic vasomotions in the forehead skin may reflect protective mechanisms in the basin of the internal carotid artery (including for the capillaries of the brain) from an excessive increase in hydrostatic pressure in conditions of increased arterial blood flow and obstructed venous outflow (myogenic autoregulation of the cerebral vascular bed). It is the myogenic tone that is the last regulatory link at the entrance to the capillary. An increase in the level of tissue perfusion in the forehead skin may be a consequence of activation of other regulatory mechanisms or a contribution to the overall spectral power of the reflected signal of the venular component (venular fullness) against the background of venous outflow difficulty from the head in HDBR conditions. The restoration of the blood perfusion level in the forehead skin on days 7, 15, and 20 of HDBR back to the initial background level is probably due to the consequence of temporary verticalization of the subjects on the eve of the study session associated with the passive orthoprobe (days 6, 14, and 19) and the LBNP test (day 19).
The detected changes (decreased perfusion and amplitude of myogenic oscillations) on days 3 and 18 may indicate the redistribution of blood from the skin of the shin and forehead into larger vessels against the background of centralization of blood flow. It was previously shown that during space flight conditions, the main volume of extracellular fluid, according to bioimpedance measurements, is observed in the abdominal region [19].
It was found that the changes in skin perfusion in the forehead and lower leg area under HDBR conditions are similar to those recorded in a study of microcirculatory tissue systems by the LDF method on the International Space Station [10]. In comparison with the LDF results under conditions of a three-week SF, indicating a slight decrease in basal perfusion in the forearm skin throughout the flight [20], our study under similar-duration conditions revealed no changes in the LDF parameter in the forearm skin.
Nevertheless, the analysis of the above functional tests showed that HDBR exposure leads to changes in the vasomotor function of the vessels of the forearm skin, which may be indicated by both a decrease in VAR and a decrease in IDP. This, hence, states a decrease in the microvascular response to both types of constrictor stimuli. The steadily reduced value of IDP throughout the entire period of HDBR indicates that the degree of shortening of smooth muscle cells during activation of the sympathetic adrenergic system decreases either due to changes in the sensitivity of myocytes to norepinephrine, or due to increased muscle tone. In this regard, it can be assumed that the tone of myocytes is increased on the forearm (the higher the initial tone, the lower the degree of muscle shortening and, consequently, the lower the amplitude of the recorded signal). However, according to the amplitude-frequency wavelet analysis, we did not register a significant decrease in the amplitude of myogenic vasomitations. The constrictor reaction in venous occlusion is based on the contraction of precapillary arterioles in response to stretching of myocytes with increasing pressure, initially in the venous region, then in the capillary and in the precapillary (Ostroumov-Baylis mechanism).
The decrease in the VAR index in HDBR conditions can be explained by the obstructed blood flow and free venous flow in the ICR system, which does not have time to fill during two minutes of occlusion. It is also impossible to exclude an increased tone of myocytes in HDBR conditions. An increase in RCC can be considered from the perspective of increasing the sensitivity of smooth muscle cells to the dilatory effect of metabolic products with temporary restriction of blood flow. At the same time, according to some literature data, the reaction to arterial occlusion may indirectly indicate a change in endothelial function [13][21]. We assumed that the increase in RCC observed in our experiment may indirectly point to a change in endothelial function, since other authors report data on impaired endothelium-dependent vasodilation (in a sample with acetylcholine) in ANH without prophylaxis in both men [22] and women [23].
CONCLUSION
Our study investigated the perfusion dynamics and regulatory mechanisms of vascular tone formation in various skin areas in healthy men under conditions of 21-day HDBR exposure. It has been shown that the skin areas of the forehead and lower extremities respond more strongly to this type of exposure from the skin microcirculation and its regulation. The effect of blood redistribution and physical inactivity is manifested in a decrease in skin perfusion against the background of increased tone of terminal arterioles and precapillary sphincters. Such dynamics may indicate the redistribution of microcirculation toward larger vessels. Despite the stability of perfusion in the forearm skin at rest, the functional tests performed showed the probability of HDBR conditions to invoke changes in vasomotor function.
Further research should address skin microhemodynamics in relation to conditions of actual weightlessness using the HDBR model.
References
1. Egorov AD, Itsekhovskii OG. Study of the cardiovascular system in long-term space flights. Space biology and aerospace medicine. 1983; 17(5):4–6 (In Russ.).
2. Blaber AP., Goswami N, Xu D. Prolonged unloading of the cardiovascular system during bedrest and spaceflight weakens neural coupling between blood pressure and heart rate. Acta Astronautica. 2022; 195:567–73. https://doi.org/10.1016/j.actaastro.2022.03.009
3. Pizzorni C, Sulli A, Smith V, Lladó A, Paolino S, Cutolo M, Ruaro B. Capillaroscopy 2016: new perspectives in systemic sclerosis. Acta Reumatol Port. 2016;41:8–14.
4. Johnson JM, Minson CT, Kellogg DL. Cutaneous Vasodilator and Vasoconstrictor Mechanisms in Temperature Regulation. Comprehensive Physiology. 2014;4(1):33–89. https://doi.org/10.1002/cphy.c130015
5. Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003; 78 (5):603–12. https://doi.org/10.4065/78.5.603
6. Fedorovich AA, Rodnenkov OV, Ageeva NV, Osyeva MK, Rogoza AN. Parameters of microcirculatory blood flow in human skin under conditions of prolonged thermal stress (model experiment). Cardiological Bulletin. 2013;1(20):7–17. EDN: RNIWAT
7. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Physiology and Pharmacology of Temperature Regulation. 2006;291:R596–R602. https://doi.org/10.1152/ajpregu.00710.2005
8. Yuan M, Custaud MA, Xu Z, Wang, J, Yuan M, Tafforin C, Treffel L, et al. Multi-system adaptation to confinement during the 180-day controlled ecological life support system (CELSS) experiment. Front. Physiol. 2019;10:575. https://doi.org/10.3389/fphys.2019.00575
9. Lloret J, Arnaud L, Gauquelin G, Ming Y, Yin X, LI Y. Cardiospace French Chinese Cooperation in Gravitational Physiology. 39th ISGP Meeting & ESA Life Sciences Meeting. Noordwijk; 2019.
10. Dunaev AV, Loktionova JI, Zharkikh EV, Fedorovich AA, Sidorov VV, Vasin AV, Dubinin IV. Investigation of blood microcirculation in microgravity with the use of portable laser Doppler flowmeters. Aerospace and environmental medicine. 2024;58(1):47–54 (In Russ.). https://doi.org/10.21687/0233-528X-2024-58-1-47-54.10.1016
11. Puchkova AA, Shpakov AV, Baranov VM, Katuntsev VP, Stavrovskaya DM, Primachenko GK, et al. General results of the 21-day head-down bedrest study without the use of countermeasures. Aerospace and environmental medicine. 2023;57(4):31–41 (In Russ.). https://doi.org/10.21687/0233-528X-2023-57-4-31-41
12. Lapitan DG, Rogatkin DA. Functional studies of the blood microcirculation system by laser Doppler flowmetry in clinical medicine: problems and prospects. The Almanac of Clinical Medicine. 2016; 44 (2):249–59 (In Russ). https://doi.org/10.18/786/2072-0505-2016-44-2-249-259
13. Sagaidachnyi A.A. Reactive hyperemia test: methods of analysis, mechanisms of reaction and prospects. Regional blood circulation and microcirculation. 2018;17(3):5–22 (In Russ.). https://doi.org/10.24884/1682-6655-2018-17-3-5-22
14. Nosovsky AM, Popova OV, Smirnov YuI. State-of-the art technologies of medical data statistical analysis and methods of graphic presentation. Aerospace and environmental medicine. 2023;57(5):149–54 (In Russ.). https://doi.org/10.21687/0233-528X-2023-57-5-149-154
15. Grigoriev AI, Kozlovskaya IB. Annual head-down bed rest (HDBT) is a physiological model of interplanetary space flight. Moscow: Russian Academy of Sciences; 2018 (In Russ.). EDN: MWLHGQ
16. Rudenko EA, Baranov MV, Zakharov SYu. Investigation of the parameters of central and peripheral hemodynamics during prolonged stay in conditions of orthostatic and antiorthostatic hypokinesia. Aerospace and environmental medicine. 2019;53(7):40–7 (In Russ.). https://doi.org/10.21687/0233-528X-2019-53-7-40-47
17. Breit, GA., Watenpaugh DE., Ballard RE., Hargens AR. Acute cutaneous microvascular flow responses to whole-body tilting in humans. Microvascular Research. 1993;46:351–8. https://doi.org/10.1006/mvre.1993.1058
18. Kurazumi T, Kato T, Konishi T, Ogawa Y, Iwasaki K. Alteration in facial skin blood flow during acute exposure to -10 and -30° head-down tilt in young human volunteers Experimental Physiology. 2022;107(12):1432–9. https://doi.org/10.1113/EP090734
19. Noskov VB, Nichiporuk AI, Vasilyeva GYu, Smirnov YuI. The composition of the human body during prolonged stay in zero gravity. Aerospace and environmental medicine. 2015;49(1):19–25 (In Russ.). EDN: TJUDQH
20. Navasiolava N, Yuan M, Murphy R, Robin A, Coupé M, Wang L, Custaud MA. Vascular and Microvascular Dysfunction Induced by Microgravity and Its Analogs in Humans: Mechanisms and Countermeasures. Frontiers in Physiology. 2020. https://doi.org/10.3389/fphys.2020.00952
21. Vlasov TD, Nesterovich II, Shimanski DA. Endothelial dysfunction: from the particular to the general. Return to the «Old Paradigm»? Regional blood circulation and microcirculation. 2019;18(2):19–27 (In Russ.). https://doi.org/10.24884/1682-6655-2019-18-2-19-27
22. Coupé M, Fortrat JO, Larina I, Gauquelin-Koch G, Gharib C, Custaud MA. Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respiratory Physiology & Neurobiology. 2009;169S:10–12. https://doi.org/10.1016/j.resp.2009.04.009
23. Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293(5): H3159-H3164
About the Authors
D. V. PashkovaRussian Federation
Daria V. Pashkova
Moscow
J. A. Popova
Russian Federation
Julia A. Popova
Moscow
A. A. Fedorovich
Russian Federation
Andrey A. Fedorovich
Moscow
A. V. Shpakov
Russian Federation
Alexey V. Shpakov
Moscow
Supplementary files
Review
For citations:
Pashkova D.V., Popova J.A., Fedorovich A.A., Shpakov A.V. Regional cutaneous blood flow in healthy subjects under conditions of 21-day head-down bed rest. Extreme Medicine. 2025;27(1):124-130. https://doi.org/10.47183/mes.2025-267

















