Scroll to:
Functional electrical stimulation for gait correction in the early recovery phase after ischemic stroke
https://doi.org/10.47183/mes.2025-268
Abstract
Introduction. Gait dysfunction is a complication of acute cerebrovascular accidents, which is biomechanically manifested as reduced speed and asymmetry in spatiotemporal and kinematic parameters. These impairments can be corrected using functional electrical stimulation (FES) of muscle contraction; however, the available literature primarily describes its application during the late recovery phase of stroke.
Objective. Evaluation of the potential of multichannel FES for gait recovery in early post-stroke rehabilitation.
Materials and methods. The study included 11 patients (2 females and 9 males) aged 46–66 years in the early recovery period after an ischemic stroke (time since stroke onset was 69.1 ± 52.0 days) and 34 healthy subjects (18 females and 16 males) as a control group. The lower limb muscle strength and tone were assessed using the Medical Research Council Scale for Muscle Strength and the modified Ashworth scale, respectively. Gait function was evaluated using the Dynamic Gait Index, Hauser Ambulation Index, Timed-Up-and-Go test, and 10-Meter Walk test. Gait pattern function (b770), obstacle negotiation (d4551), and short-distance walking (d4500) were also examined. All patients underwent a FES therapy course (mean number of sessions — 10.8). Clinical and biomechanical examinations were performed before and after the FES therapy course. Biomechanical gait analysis was conducted using a Stadis system (Neurosoft, Russia). Statistical analysis was performed using the Statistica 12.0 software.
Results. The conducted clinical evaluation demonstrated a minor yet statistically significant functional improvement in post-treatment testing. An increase in the scores of Dynamic Gait Index and 10-Meter Walk test was observed. A decrease in the values of Hauser Index values and the completion time of Timed- Up-and-Go test, as well as in domains (d770) and (d4500), was noted. Gait function showed improvement. The values of walking speed (p < 0.05), double support time on the paretic side (p < 0.05), and m. gastrocnemius activity on both the paretic and unaffected sides (p < 0.05) increased.
Conclusions. The observed changes in gait function were typical of hemiparesis. During the FES therapy course, the patients showed no negative reactions. The clinical and biomechanical gait functions of patients showed minor but positive changes during the FES therapy course. Among biomechanical parameters, the amplitude of the gastrocnemius muscle course on the paretic side significantly increased, which is one of the FES target parameters. Short courses of multichannel FES can be applied in this patient category; however, their effectiveness is insufficient. Approaches to improving the FES effectiveness require further investigation.
Keywords
For citations:
Skvortsov D.V., Grebenkina N.V., Klimov L.V., Kaurkin S.N., Bulatova M.A., Ivanova G.E. Functional electrical stimulation for gait correction in the early recovery phase after ischemic stroke. Extreme Medicine. 2025;27(3):417-428. https://doi.org/10.47183/mes.2025-268
INTRODUCTION
Acute cerebrovascular accident (ACVA) is the second leading cause of death and one of the main causes of disability worldwide [1][2]. The incidence of strokes and the costs associated with necessary rehabilitation measures have been growing globally, including due to persistent health impairment experienced by a significant proportion of ACVA survivors [3].
The complications of ACVA can be distinguished into motor [4], cognitive [5], and sensory impairments [6]. One serious complication of motor disorders сonsists in an increased risk of falls [7] due to dorsoflexor weakness and the appearance of foot drop in the paretic lower limb [8]. A slow walking speed and asymmetry of lower limb movements are often observed, associated with reduced range of motion in the joints and the need to swing the leg sideways [9][10][11][12]. In particular, spatial asymmetry is related to step length changes [13][14].
Given the urgency of prompt restoration of motor functions in ACVA patients, improved rehabilitation methods are increasingly attracting the research attention. One such approach is functional electrical stimulation (FES) of muscle contraction.
Moe et al. described the FES method primarily in the context of performing a specific functional task [15], particularly walking [16]. A number of studies reported the effectiveness of FES in correcting typical gait changes in hemiparesis. However, the mechanism of this effect and the system for evaluating the results remain unclear. Most FES studies use changes in walking speed and muscle strength as criteria [17][18]. Although being clinically significant, these criteria do not provide a detailed biomechanical understanding.
The authors [19] investigated the direct effects of FES on the gluteus medius and tibialis anterior muscles in post-stroke patients, including those using walking aids, and noted the importance of analyzing movements not only of the affected limb but also of the unaffected limb. Despite the findings, the researchers could not clarify the etiology of the increased step length in the unaffected limb. Another study [20] demonstrated the possibility of correcting knee hyperextension and foot drop with FES; however, the authors emphasized the need for further methodological development of this approach. Unfortunately, the current literature does not address the use of FES in patients during the early recovery period after a stroke.
In this study, we set out to assess the feasibility and to evaluate the outcomes of multichannel FES applied in patients during the early recovery period after a stroke for gait function correction.
MATERIALS AND METHODS
The study was conducted at the Scientific Research Center for Medical Rehabilitation of the Federal Center for Brain and Neurotechnologies from April to December 2024.
The study included patients with hemiparesis in the early recovery period after a first-ever ischemic stroke (< 180 days) in the middle cerebral artery territory, aged under 75 years, capable of independent ambulation (walking) without assistance, including with the use of walking aids (cane).
The exclusion criteria were cognitive impairments preventing patients from understanding instructions; sensorimotor aphasia; decompensated somatic pathology; diseases of the central and peripheral nervous system (except stroke) accompanied by neurological deficits (sequelae of trauma, tumors, polyneuropathies, etc.); orthopedic pathology (joint deformities, contractures, amputations, etc.); history of epileptic activity; skin diseases with rashes in electrode placement areas; patient refusal to participate.
Following screening, 11 patients (2 females and 9 males) aged 46 to 66 years (mean age 57.6 ± 8.0 years) were enrolled. Right-sided hemiparesis was observed in 4 participants. The mean time since stroke was 69.1 ± 52.0 days. The mean body mass index in the group was 24.9 kg/m2.
Additionally, 34 healthy participants (18 females and 16 males) were included as a control group. The mean age of participants was 29.8 years, with a mean body mass index of 20.6 kg/m2.
Clinical Status Assessment Methodology
For assessing the clinical status of patients, the following scales and scoring systems were used:
- Lower limb muscle strength was evaluated using the Medical Research Council Scale for Muscle Strength [21];
- Lower limb muscle tone was assessed with the Modified Ashworth Scale (mAS) [22].
The following instruments were applied for gait function evaluation: Dynamic Gait Index (DGI) [23], Hauser Ambulation Index [24], Timed-Up-and-Go Test (TUG) [25], 10 Meter Walk Test (10MWT) [26].
Health impairments and patient capabilities were assessed within the “Activity and Participation” domains of the International Classification of Functioning, Disability and Health [27][28]: gait pattern function (b770), negotiating obstacles (d4551), short-distance walking (d4500).
Gait Function Assessment Methodology
Study Procedure
All patients underwent preliminary biomechanical gait analysis using a Stadis system (Neurosoft, Russia). Spatiotemporal and kinematic gait parameters were recorded using inertial sensors secured with elastic cuffs at the sacrum level and on both lower limbs: on the lateral surface of the mid-thigh, at the lateral malleolus of the ankle joint, and on the dorsal foot surface. Simultaneously, electromyographic (EMG) activity of lower limb muscles was recorded (each sensor included two EMG channels) via electrodes placed at the mid-length of:
- quadriceps femoris,
- hamstrings (biceps femoris, m. semitendinosus, m. semimembranosus),
- tibialis anterior,
- gastrocnemius (both heads).
During testing, patients walked at a self-selected pace along an 8.5-m straight path with turns at the end. Biomechanical data were recorded until 30 gait cycles had been achieved. The software automatically excluded unstable steps (turns, stumbling, acceleration/deceleration). The output included:
- spatiotemporal gait cycle parameters,
- kinematic data as joint angle curves (flexion/extension during the gait cycle) for hip, knee, and ankle joints,
- muscle EMG activity profiles (envelope EMG).
The first biomechanical assessment was performed for both patient and healthy control groups (baseline); the second assessment was conducted only for patients after FES therapy.
Recorded Parameters
Temporal (gait cycle [GC] in sec; others as % of GC):
- stance phase (ST, %),
- single support phase (SS, %),
- double support phase (DS, %),
- the beginning of the terminal double limb stance phase (BTDLS, %).
Spatial:
- foot clearance (cm),
- circumduction (cm),
- walking speed (km/h).
Kinematic: angular range of motion (maximum flexion/extension, °) with temporal phase (% of GC).
Hip joint (H):
- amplitude and phase of initial flexion (Ha1 and Hx1, respectively),
- extension during late-stance (Ha2, Hx2),
- flexion during swing (Ha3, Hx3).
Knee joint (K):
- initial amplitude (K0),
- amplitude and phase of first flexion (Ka1, Kx1),
- amplitude and phase of first extension (Ka2, Kx2),
- amplitude and phase of second flexion (Ka3, Kx3).
Ankle joint (AJ):
- initial amplitude (A0),
- amplitude and phase of first dorsiflexion (Aa1, Ax1),
- amplitude and phase of first plantarflexion (Aa2, Ax2),
- amplitude and phase of second dorsiflexion (Aa3, Ax3),
- amplitude and phase of second plantarflexion (Aa4, Ax4).
EMG activity (peak amplitude [μV] and phase [%GC]):
- tibialis anterior (TA): two peaks (TAa1, TAa2) with phases (TAx1, TAx2),
- gastrocnemius (GSC): one peak (GSCa) and phase (GSCx),
- quadriceps femoris (QF): two peaks (QFa1, QFa2) with phases (QFx1, QFx2), respectively
- hamstrings (HM): one peak (HMa) and phase (HMx).
Recorded goniograms and envelope EMG (muscle activation profile) parameters are illustrated in Fig. 1.
Functional Electrical Stimulation (FES) Methodology
For the FES procedure, we used stimulation devices from a Stedis system (Neurosoft, Russia), and FIAB stimulation electrodes (Italy). The devices were secured with the same elastic cuffs as those used for biomechanical gait analysis, positioned on: the sacrum, thighs, and external malleoli. Stimulation electrodes were applied to the muscles of the paretic limb at the upper and lower thirds of mm. quadriceps femoris, tibialis anterior, gastrocnemius and hamstring (Fig. 2).
At the next stage, the current intensity was adjusted based on two criteria: test stimulation had to produce visible muscle contraction, while the patient’s sensations had to remain below their pain threshold. The current intensity (stimulation strength) was set at the beginning of each session for each stimulated muscle. The current frequency and pulse duration parameters remained unchanged, i.e., 50 Hz and 200 ms, respectively.
After determining the current intensity, the system was calibrated and the training was initiated. Patients walked in a straight line at a self-selected pace, making turns at the end of the path and continuing to walk. Electrical pulses were delivered to the muscles at specific points in the gait cycle corresponding to the physiological peak of muscle bioelectric activity during walking in healthy individuals. Specifically:
- for quadriceps femoris — pulses were delivered at the beginning of the stance phase and at the end of the swing phase;
- for hamstring — at the beginning of the stance phase and the end of the swing phase;
- for tibialis anterior — at the beginning of the stance phase and in the middle of the swing phase;
- for gastrocnemius — in the middle of the gait cycle.
The patient continued walking for 30 min, after which the training session ended. The procedure was stopped earlier if subjective complaints appeared (dizziness, fatigue) or at the patient’s request. Procedures were performed daily, five times per week. The course duration was determined by the patients’ hospital stay and averaged 10.8 procedures. The average procedure duration was 25.5 min.
Data Statistical Processing
For statistical data processing, we used the Statistica 12.0 software (StatSoft, Tulsa, USA). The normality of quantitative parameter distributions was assessed using the Shapiro–Wilk test, which showed non-normal distributions (p < 0.05); therefore, all data were presented as medians with first and third quartiles Me [Q1; Q3]. To compare walking parameters in patients before and after the FES course, we used the Wilcoxon test. To compare walking parameters between the patient and control groups, we applied the Mann–Whitney U-test. A p-value < 0.05 was considered statistically significant.
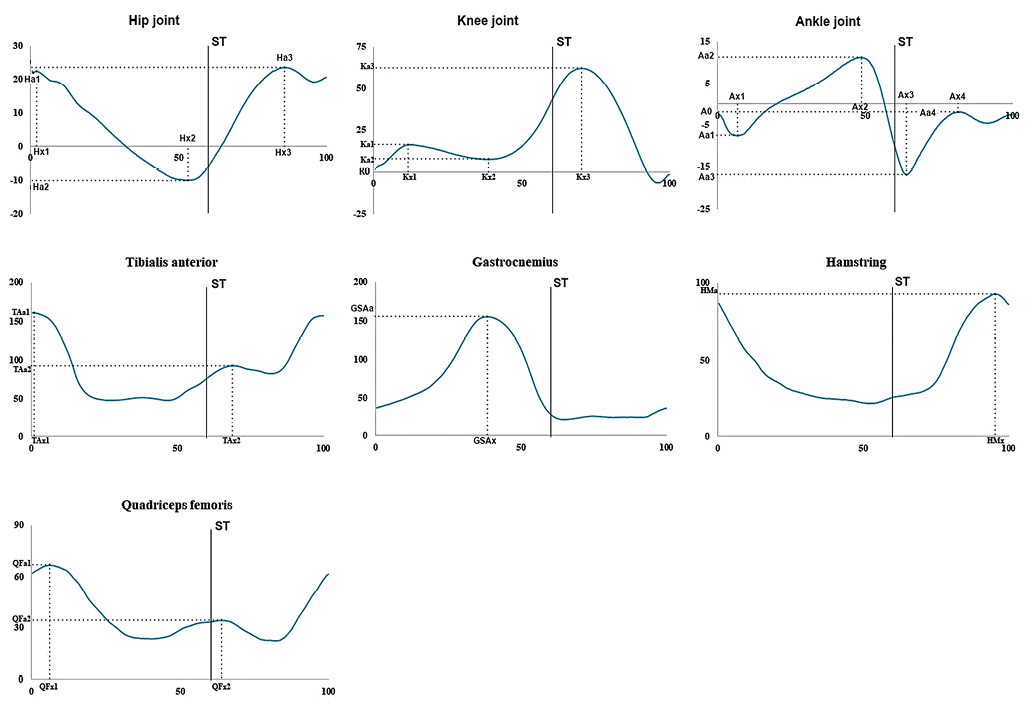
Figure prepared by the authors using their own data
Fig. 1. Parameters analyzed in goniograms curves and electromyographic (EMG) muscle activity profiles: vertical scale for goniograms (hip, knee, and ankle joints) — in degrees; for muscle activity profiles (m. tibialis anterior, m. gastrocnemius, m. quadriceps femoris, hamstring) — in microvolts; horizontal scale — in percentage of the gait cycle
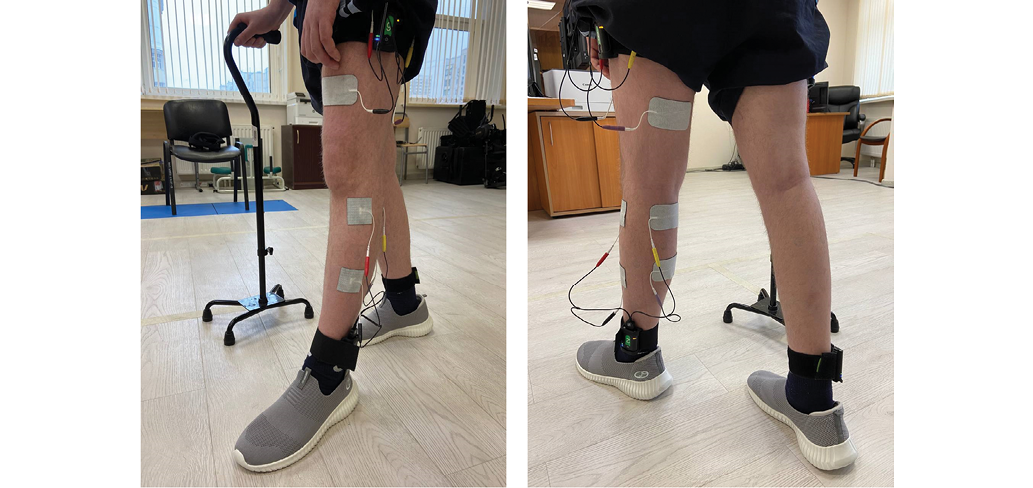
Photo taken by the authors
Fig. 2. Placement of stimulation electrodes and devices on the patient’s lower limb: the electrodes were placed on the hemiparetic side, while the devices were attached to both legs to record biomechanical parameters during stimulation
RESULTS
Clinical parameters
The comparison of clinical characteristics in the patient group before and after the FES course revealed statistically significant changes indicating improved walking function (Table 1):
- Dynamic Gait Index increased by 3 points;
- Hauser Ambulation Index decreased by 1 point;
- Timed-Up-and-Go test improved by 7 s;
- 10-Meter Walk test speed increased by 0.15 m/s.
ICF (International Classification of Functioning) domains:
- gait pattern function (b770) decreased by 1 point,
- short-distance walking (d4500) decreased by 1 point.
Spatial and temporal parameters
The comparison of parameters before and after the FES course revealed the following statistically significant changes (Table 2):
- Increased double support time on the paretic side,
- Decreased double support time on the unaffected side,
- Improved walking speed.
The comparison of pre- and post-FES parameters with control group values revealed the following statistically significant differences:
- extended gait cycle duration (GC),
- increase in the stance phase (ST) duration on the unaffected side,
- increased paretic-side single support (SS) phase,
- increase in double support (DS) phase bilaterally,
- earlier beginning of the terminal double limb stance phase (BTDLS) on the paretic side,
- delayed beginning of the terminal double limb stance phase (BTDLS) on the unaffected side,
- reduced foot clearance on the paretic side,
- increased circumduction on the paretic side,
- significantly slower walking speed in the patient group compared to healthy controls.
Kinematic parameters
The comparison of pre- and post-FES parameters revealed the following statistically significant (p < 0.05) changes (Table 3):
- earlier Hx3 on the paretic side,
- increased Ha2 on the unaffected side,
- delayed Kx1 on the unaffected side,
- delayed Ax1 on the unaffected side,
- increased Aa3 on the unaffected side.
The comparison of pre-FES patient parameters with healthy controls revealed statistically significant differences (p < 0.05). Thus, the patients demonstrated reduced amplitude of initial hip flexion (Ha1) on the paretic side; earlier onset of this flexion (Hx1) on the unaffected side; decreased extension amplitude (Ha2) bilaterally with earlier phase onset (Hx2) on the paretic side and delayed onset on the unaffected side; reduced swing-phase flexion amplitude (Ha3) on the paretic side with increased amplitude on the unaffected side; and delayed onset of this flexion phase (Hx3) on the unaffected side.
In the knee joint, the analysis revealed significant kinematic alterations, i.e., reduced amplitude of first flexion (Ka1) on the paretic side accompanied by earlier onset of this flexion phase (Kx1) bilaterally; decreased extension amplitude (Ka2) with premature phase initiation (Kx2) on the paretic side; reduction in flexion amplitude (Ka3) on the paretic limb coupled with delayed flexion onset (Kx3) on the unaffected side.
In the ankle joint, earlier onset of the first extremum phase (Ax1) bilaterally and delayed initiation of full flexion (Ax2) on the unaffected side; reduced amplitude (Aa3) bilaterally with delayed phase onset (Ax3) on both sides; increased amplitude (Aa4) bilaterally and delayed initiation of its phase (Ax4) on the paretic side were noted.
The comparative analysis of kinematic parameters in post-FES patients versus healthy controls revealed the following statistically significant changes (p < 0.05):
Hip joint
- decreased amplitude of initial flexion (Ha1) on paretic side,
- decreased extension amplitude (Ha2) bilaterally,
- earlier extension phase onset (Hx2) on paretic side,
- delayed extension phase onset (Hx2) on unaffected side,
- decreased flexion amplitude (Ha3) on paretic side,
- increased flexion amplitude (Ha3) on unaffected side.
Knee joint
- decreased initial flexion amplitude (Ka1) on paretic side,
- earlier initial flexion onset (Kx1) on paretic side,
- decreased amplitudes (Ka2 and Ka3) on paretic side,
- delayed termination of second flexion phase (Kx3) on unaffected side.
Ankle joint
- increased first amplitude (Aa1) on paretic side,
- earlier first phase onset (Ax1) on paretic side,
- decreased second amplitude (Aa2) on paretic side,
- delayed second phase onset (Ax2) on unaffected side,
- decreased third amplitude (Aa3) bilaterally,
- delayed third phase onset (Ax3) bilaterally,
- increased fourth amplitude (Aa4) bilaterally,
- delayed fourth phase onset (Ax4) on paretic side.
Electromyographic parameters
The comparison of muscle bioelectrical activity profiles before and after the FES course revealed two statistically significant changes (p < 0.05): an increase in the peak activity of the gastrocnemius muscle was observed for both the paretic and unaffected sides (Table 4).
The comparative analysis of pre-FES electromyographic parameters between patients and healthy controls revealed the following statistically significant differences (p < 0.05):
m. tibialis anterior
- decreased TAa1 amplitude on paretic side,
- delayed TAx1 onset bilaterally,
- reduced TAa2 amplitude on paretic side,
- earlier TAx2 onset on paretic side,
m. gastrocnemius
- reduced GSCa amplitude on paretic side,
- earlier GSCx onset on paretic side,
- delayed GSCx onset on unaffected side,
m. quadriceps femoris
- increased QFa1 amplitude on unaffected side,
- delayed QFx1 onset bilaterally,
- increased QFa2 amplitude on unaffected side,
Hamstring muscles
- reduced HMa amplitude on paretic side,
- earlier HMx onset on paretic side.
The comparative analysis of post-FES electromyographic profiles between patients and healthy controls revealed the following statistically significant differences (p < 0.05):
m. tibialis anterior:
- decreased TAa1 amplitude on paretic side,
- increased TAa1 amplitude on unaffected side,
- delayed TAx1 onset bilaterally,
- reduced TAa2 amplitude on paretic side,
- earlier TAx2 onset on paretic side,
m. gastrocnemius:
- reduced GCa amplitude on paretic side,
- quadriceps femoris:
- delayed QFx1 onset on unaffected side,
Hamstring muscles:
- decreased HMa amplitude on paretic side,
- increased HMa amplitude on unaffected side.
Table 1. Clinical parameters before and after the functional electrical stimulation (FES) course
|
Clinical Parameter |
Before FES |
After FES |
|
Lower-extremities muscle strength, score |
3 |
3 |
|
Clinical scales and tests |
||
|
Lower-extremities muscle tone on Modified Ashworth Scale, score |
1–2 |
1–2 |
|
Dynamic Gait Index |
16 [ 14; 17] |
19* [ 18; 20] |
|
Hauser Ambulation Index |
4 [ 3; 4] |
3* [ 3; 4] |
|
Timed-Up-and-Go test, s |
32 [ 25; 36] |
25* [ 19; 30] |
|
10-Meter Walk test, m/s |
0.75 [ 0.7; 0.8] |
0.9* [ 0.85; 1] |
|
ICF categories |
||
|
b770 — gait pattern functions |
2 [ 2; 3] |
1* [ 1; 2] |
|
d4551 — obstacle negotiation |
2 [ 1; 2] |
1 [ 1; 2] |
|
d4500 — short-distance walking |
2 [ 1; 2] |
1* [ 0; 1] |
Table compiled by the authors based on their own data
Note: * — statistically significant changes, p < 0.05.
Тable 2. Spatiotemporal parameters before and after the functional electrical stimulation (FES) course
|
Parameter |
Before FES course |
After FES course |
Control group |
||
|
Paretic side |
Unaffected side |
Paretic side |
Unaffected side |
||
|
GC, s |
1.6 [ 1.5; 2]* |
1.6 [ 1.4; 1.9]* |
1.5 [ 1.4; 2]* |
1,5 [ 1,4; 2]* |
1,1 [ 1,1; 1,2] |
|
ST (%) |
63.3 [ 60.8; 64.5] |
74.2 [ 69.1; 78]* |
62.1 [ 59.9; 65] |
71,8 [ 67,9; 78,2]* |
63,1 [ 62,4; 64,4] |
|
SS (%) |
26.3 [ 22.2; 31.2]* |
36.9 [ 35.9; 39.5] |
27.6 [ 21.5; 31.7]* |
37,8 [ 35,2; 39,7] |
36,9 [ 35,7; 37,9] |
|
DS (%) |
34.5 [ 30.6; 43]* |
34.8 [ 30.7; 42.8]* |
35 [ 27.6; 40.8]* # |
34,4 [ 28,2; 41,4]* # |
26,1 [ 24,6; 28,1] |
|
BTDLS (%) |
41.6 [ 40.8; 45.8]* |
57.1 [ 53.5; 60]* |
42.8 [ 40; 45.6]* |
56,4 [ 54,1; 60,1]* |
49,9 [ 49,6; 50,3] |
|
Foot clearance (cm) |
8 [ 7; 12]* |
13 [ 11; 15] |
9 [ 7; 12]* |
14 [ 11; 14] |
13,5 [ 12; 15] |
|
Circumduction (cm) |
4 [ 3; 6]* |
2 [ 2; 4] |
4 [ 3; 6]* |
2 [ 2; 3] |
3 [ 2; 4] |
|
Walking Speed (km/h) |
1.7 [ 1.2; 2.5]* |
2.2 [ 1.3; 2.4]*# |
4.3 [ 4; 5] |
||
Table compiled by the authors based on their own data
Note: * — significant differences versus controls, p < 0.05; # — pre-post differences in ipsilateral parameters reached statistical significance, p < 0.05; GC — gait cycle; ST — stance phase; SS — single support phase; DS — double support phase; BTDLS — the beginning of the terminal double limb stance phase.
Table 3. Kinematic parameters before and after the functional electrical stimulation (FES) course
|
Location |
Parameter |
Before FES course |
After FES course |
Control group |
||
|
Paretic side |
Unaffected side |
Paretic side |
Unaffected side |
|||
|
Hip Joint |
Ha1 |
15* [ 9; 16] |
23 [ 19; 30] |
15* [ 10; 17] |
24 [ 20; 28] |
23 [ 20; 25] |
|
Hx1 |
2 [ 1; 5] |
1* [ 1; 2] |
3 [ 1; 7] |
2 [ 1; 5] |
2 [ 2; 3] |
|
|
Ha2 |
-6* [ -9; 1] |
-6* [ -10; -3] |
-8* [ -11; -2] |
-7*# [ -11; -3] |
-11 [ -12; -9] |
|
|
Hx2 |
50* [ 47; 55] |
59* [ 56; 64] |
50* [ 47; 52] |
61* [ 57; 66] |
53 [ 51; 55] |
|
|
Ha3 |
16* [ 11; 28] |
31* [ 26; 34] |
17* [ 16; 27] |
31* [ 25; 32] |
24 [ 22; 27] |
|
|
Hx3 |
90 [ 84; 92] |
90* [ 86; 93] |
88# [ 83; 91] |
89 [ 87; 93] |
87 [ 84; 89] |
|
|
Knee Joint |
K0 |
2 [ 0; 4] |
12* [ 8; 15] |
1 [ -3; 5] |
10* [ 7; 13] |
3 [ -1; 5] |
|
Ka1 |
10* [ 4; 12] |
14 [ 14; 20] |
10* [ 3; 11] |
16 [ 13; 19] |
17 [ 14; 19] |
|
|
Kx1 |
8* [ 7; 10] |
9* [ 7; 12] |
11* [ 8; 13] |
10# [ 7; 15] |
12 [ 12; 14] |
|
|
Ka2 |
2* [ -4; 9] |
6 [ 4; 9] |
-1* [ -4; 2] |
5 [ 2; 11] |
6 [ 4; 9] |
|
|
Kx2 |
33* [ 31; 37] |
38 [ 34; 43] |
37 [ 32; 42] |
38 [ 35; 40] |
37 [ 34; 41] |
|
|
Ka3 |
35* [ 27; 52] |
61 [ 56; 62] |
37* [ 30; 47] |
61 [ 59; 64] |
63 [ 60; 67] |
|
|
Kx3 |
70 [ 66; 73] |
79 [ 74; 83]* |
71 [ 64; 73] |
77* [ 74; 81] |
70 [ 69; 71] |
|
|
Ankle Joint |
A0 |
-9* [ -12; -2] |
-4 [ -5; -3] |
-10* [ -15; -6] |
-3 [ -4; -1] |
-3 [ -5; 0] |
|
Aa1 |
-11 [ -14; -5] |
-7 [ -9; -4] |
-14* [ -15; -13] |
-7 [ -10; -5] |
-8 [ -10; -6] |
|
|
Ax1 |
4* [ 1; 5] |
4* [ 3; 7] |
3* [ 1; 6] |
6# [ 3; 8] |
7 [ 6; 8] |
|
|
Aa2 |
9 [ 5; 14] |
10 [ 9; 12] |
8 [ 5; 12]* |
13 [ 10; 14] |
12 [ 10; 15] |
|
|
Ax2 |
49 [ 47; 51] |
58* [ 56; 60] |
48.75 [ 48; 50] |
57* [ 56; 59] |
48 [ 46; 50] |
|
|
Aa3 |
-5* [ -11; -3] |
-9* [ -18; -7] |
-10* [ -13; -7] |
-15* # [ -17; -12] |
-19 [ -22; -15] |
|
|
Ax3 |
74* [ 66; 79] |
74* [ 71; 80] |
67* [ 65; 76] |
73* [ 70; 77] |
64 [ 63; 65] |
|
|
Aa4 |
-9* [ -11; -3] |
-4* [ -10; -4] |
-9* [ -14; -5] |
-6* [ -9; -3] |
-1 [ -3; 1] |
|
|
Ax4 |
94* [ 93; 98] |
82 [ 81; 98] |
99* [ 95; 100] |
81 [ 81; 97] |
86 [ 81; 97] |
|
Table compiled by the authors based on their own data
Note: * — significant differences versus controls, p < 0.05; # — pre-post differences in ipsilateral parameters reached statistical significance, p < 0.05; Hа1 and Hа2 — amplitude and phase of initial flexion; Hа2 and Hх2 — extension during mid-stance; Hа3 and Hх3 — flexion during swing; К0 — initial amplitude of knee; Ка1 and Кх1 — amplitude and phase of initial flexion; Ка2 and Кх2 — amplitude and phase of first extension; Ка3 and Кх3 — amplitude and phase of second flexion; A0 — initial amplitude of ankle; Aа1 and Aх1 — amplitude and phase of first dorsiflexion; Aа2 and Aх2 — amplitude and phase of first plantarflexion; Aа3 and Aх3 — amplitude and phase of second dorsiflexion; Aа4 and Aх4 — amplitude and phase of second plantarflexion.
Table 4. Electromyographic parameters before and after functional electrical stimulation (FES)
|
Muscle |
Parameter |
Before FES course |
After FES course |
Control group |
||
|
Paretic side |
Unaffected side |
Paretic side |
Unaffected side |
|||
|
ТА |
TAa1 |
72* [ 33; 95] |
163 [ 134; 230] |
69* [ 58; 135] |
208* [ 178; 278] |
159 [ 118; 186] |
|
TAx1 |
58* [ 4; 60] |
10* [ 9; 28] |
60* [ 12; 60] |
20* [ 9; 26] |
1 [ 1; 2] |
|
|
TAa2 |
70* [ 58; 104] |
143 [ 118; 215] |
71* [ 60; 139] |
180 [ 136; 222] |
154 [ 116; 185] |
|
|
TAx2 |
68* [ 62; 97] |
100 [ 84; 100] |
66* [ 64; 100] |
100 [ 84; 100] |
99 [ 98; 100] |
|
|
GSС |
GSСa |
50* [ 27; 81] |
145 [ 133; 163] |
70* # [ 54; 96] |
171# [ 164; 208] |
154 [ 113; 202] |
|
GSСx |
31* [ 28; 39] |
44* [ 35; 47] |
37 [ 32; 40] |
39 [ 35; 47] |
39 [ 37; 40] |
|
|
QF |
QFa1 |
62 [ 41; 67] |
92* [ 72; 109] |
62 [ 52; 84] |
89 [ 67; 174] |
63 [ 41; 86] |
|
QFx1 |
13* [ 10; 17] |
21* [ 6; 24] |
14 [ 6; 16] |
12* [ 9; 23] |
7 [ 5; 9] |
|
|
QFa2 |
40 [ 31; 58] |
75 [ 60; 126] |
48* [ 40; 81] |
82 [ 60; 116] |
57 [ 39; 81] |
|
|
QFx2 |
100 [ 51; 100] |
97 [ 51; 100] |
100 [ 99; 100] |
95 [ 52; 100] |
100 [ 99; 100] |
|
|
HM |
HMa1 |
53* [ 43; 71] |
108 [ 83; 146] |
59* [ 40; 79] |
129* [ 115; 146] |
83 [ 62; 123] |
|
HMx1 |
13* [ 10; 19] |
26 [ 12; 56] |
14 [ 11; 25] |
45 [ 31; 65] |
92 [ 43; 95] |
|
Table compiled by the authors based on their own data
Note: * — significant differences versus controls, p < 0.05; # — pre-post differences in ipsilateral parameters reached statistical significance, p < 0.05; TA — m. tibalis anterior; GSC — m. gastrocnemius; QF — m. quadriceps femoris; HM — hamstrings.
DISCUSSION
Our study revealed minor yet characteristic gait alterations in patients with stroke-associated hemiparesis.
Following the FES therapy course, we observed:
- increased Dynamic Gait Index scores,
- improved 10-Meter Walk test performance,
- decreased Hauser Ambulation Index values,
- reduced Timed Up-and- Go (TUG) test completion time.
These outcome measures (10-Meter Walk and TUG) represent the most frequently reported FES efficacy parameters in literature [29-31], with our results being consistent with existing data. However, other studies have incorporated additional clinical measures showing more variable outcomes.
The systematic review by Wang et al. covering 14 studies with 945 hemiparetic patients demonstrated FES-induced improvements in:
- Fugl-Meyer Assessment (FMA) scores,
- Berg Balance Scale(BBS),
- 10-Minute Walk test,
- Modified Barthel Index(MBI),
- Functional Walking Test (FWT)[30].
Conversely, an eight-week FES trial (40 min/day, 5 days/week, n=92) by Matsumoto et al. failed to show statistically significant changes in 10-Meter Walk test, Fugl-Meyer Assessment, and Timed-Up-and-Go test [32].
The changes in spatiotemporal gait parameters observed in patients prior to the FES course demonstrated alterations characteristic of this post-stroke period. These included:
- increased gait cycle duration (GC),
- normal stance phase (SP) duration on the paretic side with prolonged SP on the unaffected side,
- reduced single support time (SS) on the paretic side with increased SS on the unaffected side,
- increased total double support time (DS),
- the beginning of the terminal double limb stance phase (BTDLS) timing (earlier on paretic, delayed on unaffected side),
- decreased foot clearance on the paretic side.
These biomechanical changes have been previously described [9-12] and represent typical hemiparetic gait patterns.
Following FES intervention, we observed:
- A minor yet statistically significant increase in the double support time bilaterally (p < 0.05) — a compensatory mechanism to improve body balance, as stability increases when both limbs are weight-bearing.
- Increased walking speed, correlating with findings from other studies [33][34].
As a rule, patients with hemiparesis also exhibit kinematic changes: reduced range of motion in the hip, knee, and ankle joints on the paretic side. In this case, the ankle joint is in slight extension, which reduces clearance and leads (along with other changes) to increased circumduction. The paretic side is characterized by reduced range of motion in the joints. At the same time, the healthy side is forced to compensate for the reduced activity of the paretic side. Thus, at low walking speeds, even normative kinematic parameters of the unaffected side already represent hyperfunction. The later peaks of several amplitudes on the unaffected side are the result of increased SP. The overall duration of stance phase increases, and thus the amplitude peaks also shift and occur later in time.
Following the FES course, only minor kinematic changes were observed, primarily on the unaffected side. In the available literature, FES is most commonly used for post-stroke patients with foot drop; consequently, kinematic changes are typically limited to the ankle joint. For instance, Güzel et al. described the positive effects of a four-week FES course on the ankle joint range of motion in patients during the early recovery phase after an ischemic stroke [34].
The EMG analysis revealed characteristic hemiparetic changes, including reduced activity amplitudes in all analyzed muscles on the paretic side. However, less pronounced changes were noted in QF compared to other muscle groups, both in terms of amplitude and activation profile [12][35]. This particular muscle provides knee joint stability, and significant alterations in its activity make weight-bearing on the paretic limb impossible.
The rehabilitation course resulted in only one significant change: an increased GSC amplitude was observed bilaterally. Nevertheless, GSC activity on the paretic side remained more than two times lower than on the unaffected side, both before and after FES.
Our results demonstrate that during the early recovery period after a cerebral stroke, a three-week rehabilitation course in general and with FES application in particular objectively led to minor functional improvements. The FES training was conducted daily, with patients walking the maximum duration until fatigue. Stimulation intensity was also maintained at the maximum tolerable level for each patient. According to foreign researchers, FES courses are typically conducted over longer periods [36]. However, under the conditions of our study, exceeding 10 procedures proved particularly challenging. This limitation was previously noted in our earlier research into gait restoration using biofeedback methods [11][37].
The duration of rehabilitation measures for patients with CNS impairments depends on their Rehabilitation Routing Scale (RRS) score, which reflects the degree of functional limitations and dependence on assistance for daily activities. According to the Program of State Guarantees for Free Medical Care, patients with RRS score 4 receive 14-day rehabilitation courses, while those with RRS score 5 receive 20-day courses. Typically, gait training begins for patients with RRS level 4 functional limitations, implying the actual length of rehabilitation between 10–12 days. Objective gait assessment is performed upon admission and before discharge.
Our findings indirectly confirm the insufficient duration of CNS rehabilitation courses within the current medical rehabilitation system and highlight the need for further investigation.
CONCLUSIONS
All patients included in this study demonstrated typical gait impairments associated with hemiparesis during the early recovery phase after a stroke. The administered course of multichannel FES revealed no adverse reactions. While clinical and biomechanical improvements during the FES course were modest, a statistically significant increase in gastrocnemius muscle amplitude was observed on the paretic side.
Our findings indicate that multichannel FES can be safely implemented for gait correction during early stroke recovery. However, the short 10-session daily treatment protocol failed to produce substantial functional improvements in the stimulated muscles.
Future research should focus on developing FES parameter adjustment methodologies tailored to specific biomechanical impairments, potentially enhancing therapeutic outcomes for gait rehabilitation in patients during the early recovery phase after an ischemic stroke.
References
1. Katan M, Luft A. Global Burden of Stroke. Seminars in Neurology. 2018;38:208–11. https://doi.org/10.1055/s-0038-1649503
2. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation. 2023;147:e93–621. https://doi.org/10.1161/CIR.0000000000001123
3. Donkor ES. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Research and Treatment. 2018.27;2018:3238165. https://doi.org/10.1155/2018/3238165
4. Hendricks HT, Van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: A systematic review of the literature. Archives of Physical Medicine and Rehabilitation. 2002;83:1629–37. https://doi.org/10.1053/apmr.2002.35473
5. Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314:41–51. https://doi.org/10.1001/jama.2015.6968
6. Lima DHF, Queiroz AP, Salvo G, Yoneyama SM, Oberg TD, Lima NMFV. Brazilian version of the Nottingham Sensory Assessment: validity, agreement and rehability. Brasilian Journal of Physical Therapy. 2010;14:166–75. https://doi.org/10.1590/S1413-35552010005000006
7. Schmid AA, Wells CK, Concato J, Dallas MI, Lo AC, Nadeau SE, et al. Prevalence, predictors, and outcomes of poststroke falls in acute hospital setting. Journal of Rehabilitation Research and Development. 2010;47:553–62.
8. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An Updated Definition of Stroke for the 21st Century. Stroke. 2013;44:2064–89. https://doi.org/10.1161/STR.0b013e318296aeca
9. Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function (2 nd ed.). CRC Press; 2010. https://doi.org/10.1201/9781003525592
10. Wang Y, Mukaino M, Ohtsuka K, Otaka Y, Tanikawa H, Matsuda F, et al. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. International Journal of Rehabilitation Research. 2020;43(1):69–75. https://doi.org/10.1097/MRR.0000000000000391
11. Skvortsov DV, Kaurkin SN, Ivanova GE. Targeted Biofeedback Training to Improve Gait Parameters in Subacute Stroke Patients: A Single-Blind Randomized Controlled Trial. Sensors. 2024; 24(22):7212. https://doi.org/10.3390/s24227212
12. Skvortsov DV, Grebenkina NV, Kaurkin SN, Ivanova GE. Characteristics of gait function in hemiparetic patients with subacute period of ischemic stroke: a single-center retrospective study. Physical and Rehabilitation Medicine, Medical Rehabilitation. 2024;6(3):208–19 (In Russ.). https://doi.org/10.36425/rehab634515
13. Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Archives of Physycal Medicine and Rehabilitation. 2007;88:43–9. https://doi.org/10.1016/j.apmr.2006.10.004
14. Kim CM, Eng JJ. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait and Posture. 2003;18:23–8. https://doi.org/10.1016/S0966-6362(02)00122-4
15. Moe JH, Post HW. Functional electrical stimulation for ambulation in hemiplegia. The Lancet. 1962;82:285–8.
16. Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, et al. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Physical Therapy. 2010;90(1):55–66. https://doi.org/10.2522/ptj.20090140
17. Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clinical Rehabilitation. 1997;11(3):201–10. https://doi.org/10.1177/026921559701100303
18. Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Archives of Physical Medicine and Rehabilitation. 2006;87(6):853–9. https://doi.org/10.1016/j.apmr.2006.02.026
19. Araki S, Kawada M, Miyazaki T, Nakai Y, Takeshita Y, Matsuzawa Y, et al. Effect of Functional Electrical Stimulation of the Gluteus Medius during Gait in Patients following a Stroke. Biomed Research International. 2020;2020:8659845. https://doi.org/10.1155/2020/8659845
20. Santos GF, Jakubowitz E, Pronost N, Bonis T, Hurschler C. Predictive simulation of post-stroke gait with functional electrical stimulation. Scientific Reports. 2021;11(1):21351. https://doi.org/10.1038/s41598-021-00658-z
21. Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. Journal of Rehabilitation Medicine. 2008;40(8):665–71. https://doi.org/10.2340/16501977-0235
22. Suponeva NA, Yusupova DG, Ilyina KA, Melchenko DA, Butkovskaya AA, Zhirova ES, et al. Validation of the modified Ashworth scale in Russia. Annals of Clinical and Experimental Neurology. 2020;14(1):89–96 (In Russ.). https://doi.org/10.25692/ACEN.2020.1.10
23. Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Archives of Physical Medicine and Rehabilitation. 2007;88(11):1410–5. https://doi.org/10.1016/j.apmr.2007.08.109
24. Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, threearm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. The New England Journal of Medicine. 1983;308(4):173–80. https://doi.org/10.1056/NEJM198301273080401
25. Chan PP, Tou JI, Tse MM, Ng SS. Reliability and Validity of the Timed Up and Go Test with a Motor Task in People with Chronic Stroke. Archives of Physical Medicine and Rehabilitation. 2017;98(11):2213–20. https://doi.org/10.1016/j.apmr.2017.03.008
26. Watson MJ. Refining the ten-metre walking test for use with neurologically impaired people. Physiotherapy. 2002;88(7):386–97.
27. Melnikova EV, Builova TV, Bodrova RA, Shmonin AA, Maltseva MN, Ivanova GE. Use of the international classification of functioning (icf) in outpatient and inpatient medical rehabilitation: instruction for specialists. Bulletin of Rehabilitation Medicine. 2017;6(82):7–20 (In Russ.). EDN: ZVGCHN
28. Ivanova GE, Melnikova EV, Shmonin AA, Verbitskay EV, Aronov DM, Belkin AA, et al. Application of the international classification of functioning in the process of medical rehabilitation. Bulletin of Rehabilitation Medicine. 2018;(88):2–77 (In Russ.). EDN: YOTJKP
29. Mitsutake T, Sakamoto M, Horikawa E. The effects of electromyography-triggered neuromuscular electrical stimulation plus tilt sensor functional electrical stimulation training on gait performance in patients with subacute stroke: a randomized controlled pilot trial. International Journal of Rehabilitation Research. 2019;42(4):358–64. https://doi.org/10.1097/MRR.0000000000000371
30. Wang J, Zhao L, Gao Y, Liu C, Dong X, He X. The difference between the effectiveness of body-weight-supported tread-mill training combined with functional electrical stimulation and sole body-weight-supported treadmill training for improving gait parameters in stroke patients: A systematic review and meta-analysis. Frontiers in Neurology. 2022;13:1003723. https://doi.org/10.3389/fneur.2022.1003723
31. Dujović SD, Malešević J, Malešević N, Vidaković AS, Bijelić G, Keller T, et al. Novel multi-pad functional electrical stimulation in stroke patients: A single-blind randomized study. NeuroRehabilitation. 2017;41(4):791–800.
32. Matsumoto S, Shimodozono M, Noma T, Miyara K, Onoda T, Ijichi R, et al. Effect of Functional Electrical Stimulation in Convalescent Stroke Patients: A Multicenter, Randomized Controlled Trial. Journal of Clinical Medicine. 2023;12(7):2638. https://doi.org/10.3390/jcm12072638
33. Wonsetler EC, Bowden MG. A systematic review of mechanisms of gait speed change post-stroke. Part 1: spatiotemporal parameters and asymmetry ratios. Topics in Stroke Rehabilitation. 2017;24(6):435–46. https://doi.org/10.1080/10749357.2017.1285746
34. Güzel S, Karaca Umay E, Öztürk EA, Çakci A. The efficiency of functional electrical stimulation and balance-weighted rehabilitation therapy in stroke patients with-foot-drop: a pilot study. Journal of Physical Medicine and Rehabilitation Sciences. 2022;25(1):1–10. https://doi.org/10.31609/jpmrs.2021-82149
35. Almeida ASSC, Viana da Cruz AT, Candeira SRA, Cardozo do Nascimento NI, Santar de Castro KJ, Costa de Lima R, et al. Late physiotherapy rehabilitation changes gait patterns in post-stroke patients. Biomedical Human Kinetics. 2017;9(1):14–8. https://doi.org/10.1515/bhk-2017-0003
36. Skvortsov DV, Kaurkin SN, Ivanova GE. A Study of Biofeedback Gait Training in Cerebral Stroke Patients in the Early Recovery Phase with Stance Phase as Target Parameter. Sensors. 2021;21:7217. https://doi.org/10.3390/s21217217
37. Skvortsov DV, Klimov LV, Grebenkina NV. Functional electrical stimulation method: recommended application parameters. Physical and Rehabilitation Medicine, Medical Rehabilitation. 2024;6(3):263–79. https://doi.org/10.36425/rehab635187
38.
About the Authors
D. V. SkvortsovRussian Federation
Dmitry V. Skvortsov, Dr. Sci. (Med.), Professor
Moscow
N. V. Grebenkina
Russian Federation
Natalya V. Grebenkina
Moscow
L. V. Klimov
Russian Federation
Leonid V. Klimov, Cand. Sci. (Med.)
Moscow
S. N. Kaurkin
Russian Federation
Sergey N. Kaurkin, Cand. Sci. (Med.)
Moscow
M. A. Bulatova
Russian Federation
Mariya A. Bulatova, Cand. Sci. (Med.)
Moscow
G. E. Ivanova
Russian Federation
Galina E. Ivanova, Dr. Sci. (Med.), Professor
Moscow
Supplementary files
Review
For citations:
Skvortsov D.V., Grebenkina N.V., Klimov L.V., Kaurkin S.N., Bulatova M.A., Ivanova G.E. Functional electrical stimulation for gait correction in the early recovery phase after ischemic stroke. Extreme Medicine. 2025;27(3):417-428. https://doi.org/10.47183/mes.2025-268

















