Scroll to:
Role of regulatory T lymphocytes in the formation of immunosuppressive microenvironment in glioblastoma
https://doi.org/10.47183/mes.2025-299
Abstract
Introduction. Being the most common tumor of the central nervous system with an extremely unfavorable prognosis, glioblastoma remain to be a major health issue. Conventional neuro-oncological strategies demonstrate insufficient effectiveness, which requires the development of improved approaches.
Objective. Analysis of the mechanisms of functioning of regulatory T lymphocytes (Treg) in the tumor microenvironment as a potential target for therapy, as well as identification of promising therapeutic methods to reduce the suppressive effect of regulatory T lymphocytes in glioblastoma.
Discussion. The resistance of glioblastoma against antitumor immunity and the low effectiveness of some types of treatment is largely related to the immunosuppressive microenvironment of the tumor, the key components of which are Treg. Tregs suppress the antitumor response through the secretion of anti-inflammatory cytokines, perforins, and granzymes, as well as the expression of inhibitory molecules. Drugs that selectively affect the metabolic pathways of activation, differentiation, and migration of regulatory T cells can reduce their activity and total number in the microenvironment.
Conclusions. Tregs can act as a target for therapy aimed at suppressing the immunosuppressive microenvironment of the tumor, reducing the activity and progression of glioblastoma. New targeted therapeutical approaches may supplement the existing standards of glioblastoma treatment.
Keywords
For citations:
Yanysheva E.P., Baklaushev V.P., Yusubalieva G.M. Role of regulatory T lymphocytes in the formation of immunosuppressive microenvironment in glioblastoma. Extreme Medicine. 2025;27(2):183-190. https://doi.org/10.47183/mes.2025-299
INTRODUCTION
Glioblastoma is the most common and aggressive brain tumor characterized by an extremely high mortality rate with the median survival rate of about 13.5 months and the overall five-year survival rate of about 5.8% [1].
According to the WHO classification of 2021, glioblastoma is categorized as diffuse glioma grade IV. The primary form of glioblastoma, which occurs de novo, is differentiated from the secondary form, which develops as a result of progression of gliomas of a lower grade of malignancy (Grade II and III). At the same time, the primary type characterized by high invasiveness and rapid development is more common (up to 90% of the total number of cases) [2].
Factors that can trigger the development of malignant brain gliomas include genetic aberrations, viral infections (cytomegalovirus, herpes, etc.), and ionizing radiation, as well as a history of Turcot syndrome, neurofibromatosis type 1 and 2, or tuberous sclerosis [3]. At the same time, the risk of disease development increases with age due to a decrease in the effectiveness of DNA repair processes and the weakening of the immune response [4].
Surgical resection of the tumor, radiotherapy, and chemotherapy with temozolomide are used as treatment standards in glioblastoma patients. Surgical intervention is complicated by the invasive growth of glioblastoma, which prevents complete excision of the pathological tissue and subsequently leads to relapses of the disease. The prognosis for each individual patient is different, depending on various factors (neoplasm location, tumor subtype, diagnosis time, therapy initiation, etc.). In many cases, chemo- and radiotherapy is accompanied by the formation of resistance [5]. Overall, the standard therapy currently used shows low effectiveness, leading to negative side effects and relapses [6]. Moreover, in case of recurrence, the tumor runs a more aggressive course and shows increased therapeutic resistance [7].
The relatively high resistance of glioblastoma against various therapeutical strategies is caused by the tumor heterogeneity and the immunosuppressive microenvironment [8]. Therefore, new treatment methods that account for the characteristic features of glioblastoma are required. In this regard, immunotherapy has great potential [9] due to the possibility of modulating — either directly or indirectly — the immune response, stimulating the patient’s natural antitumor immunity and reducing pronounced immunosuppression in the glioma focus to increase the effectiveness of other types of treatment as part of combination therapy.
In this study, we aim to analyze the mechanisms of functioning of regulatory T lymphocytes (Treg) in the tumor microenvironment as a potential target for therapy, as well as to identify promising therapeutic methods for reducing the suppressive effect of regulatory T lymphocytes in glioblastoma.
MATERIALS AND METHODS
The literature search was conducted through the PubMed, Google Academy, and eLibrary databases using the following query keywords: glioblastoma, glioma, regulatory T lymphocytes, immunosuppression, microenvironment, and immunotherapy. The search depth was five years.
RESULTS AND DISCUSSION
Immunosuppressive microenvironment in glioblastoma
The development of glioblastoma is associated with the development of a tumor microenvironment (TME), which plays an important role in neovascularization initiating, progression, invasion, and metastasis of glioma [10]. As a result of this process, a complex heterogeneous system is formed, consisting of the tumor cells themselves, as well as the extracellular matrix, fibroblasts, endotheliocytes, pericytes, immune cells, and signaling molecules secreted by these cells [11]. According to [12], the TME components interact with one another and tumor cells through intercellular contacts and the secretion of various cytokines, chemokines, and growth factors.
The research team [13] noted that glioblastoma significantly affects immune cells and models their phenotype by secreting a range of biologically active molecules. In turn, the immune cells of the microenvironment maintain a high level of immunosuppression in the glioma microenvironment, which contributes to tumor progression.
The focus of glioblastoma contains immune cells whose function is inflammation and antitumor response: cytotoxic T lymphocytes (CTL), natural killers, T-helpers, dendritic cells, B lymphocytes, neutrophils, monocytes, and M-1 polarized macrophages. Tumor infiltration by effector cells has a positive prognostic value in glioblastoma [14]. However, the cells present in TME either show reduced antitumor activity or acquire a pro-tumor phenotype under the influence of glioma.
The TME contributes to the successful escape of the tumor from immunological surveillance, leading to suppression of activation and proliferation of cytotoxic T lymphocytes and NK cells, B lymphocytes, disruption of the tumor antigen presentation on the major histocompatibility complex (MHC) of dendritic cells, and the involvement of regulatory T cells in the microenvironment [15]. The lack of a sufficient level of antigen presentation associated with glioblastoma naturally leads to a low effectiveness of the adaptive immune response.
The cells that mainly provide immunosuppression in the tumor microenvironment are tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Treg) [16, 17]. Tumor-associated macrophages, including brain microglia and macrophages of peripheral origin, are the most numerous non-tumor populations in the TME in glioblastoma. The macrophage population exhibits plasticity: cells are capable of polarizing into both pro-inflammatory and anti-inflammatory phenotypes [18].
Suppressor cells of myeloid origin are a heterogeneous population of myeloid progenitor cells at different stages of differentiation, which cause inhibition of the activity of cytotoxic T lymphocytes, suppression of the function of NK cells, macrophages, and dendritic cells, as well as induction of regulatory T and B lymphocytes in the TME [19].
Regulatory T lymphocytes are the main cell population, on the one hand, supporting the immune system homeostasis, and, on the other, playing a key role in avoiding glioblastoma from the immune response. Thus, although regulatory T cells are of interest as a target for the treatment of malignant gliomas, the non-selective effects on the Treg population are associated with numerous side effects.
In the glioblastoma microenvironment, MDSC, TAM, and Treg enter synergy, complementing and enhancing the pro-tumor effects of one another. Regulatory T lymphocytes stimulate the polarization of TAMs, which in turn support the Treg suppressive activity [20]. Tregs also enhance the expansion and inhibitory function of suppressor cells of myeloid origin; MDSCs promote proliferation and induction of regulatory T cells [21].
The researchers in [22] investigated the role of regulatory B lymphocytes and regulatory NK cells as components of the immunosuppressive microenvironment. Regulatory B cells perform the immunoregulation functions through cytokine secretion and intercellular contacts. In the tumor microenvironment, regulatory B cells inhibit effector T lymphocytes, induce Treg activation, and affect other TME-infiltrating cells such as MDSCs, NK cells, and macrophages [23]. NK cells in the tumor microenvironment can perform a regulatory function, influencing the maturation of dendritic cells and leading to a decrease in CTL activation [24].
Population of regulatory T lymphocytes
Regulatory T cells are a subpopulation of CD4+ T lymphocytes, which control the duration of immune response and maintain dominant immunological tolerance to their own antigens. Disruption of the normal Treg functioning plays an important role in the pathogenesis of the graft-versus-host reaction, i.e., in autoimmune, allergic, and oncological diseases [25].
Regulatory T lymphocytes have a fairly wide repertoire of T cell receptor (TCR) specificity, predominantly recognizing their own peptides. Most Tregs are formed in the thymus as functionally mature T lymphocytes (natural Tregs), while a smaller part is induced from naive T cells after antigen-dependent differentiation in the periphery (adaptive Tregs) [26]. The population of natural regulatory T lymphocytes provides tolerance to autoantigens, while adaptive Tregs limit inflammation during infection and suppress the pathological immune response associated with transplantation and allergic conditions.
The Treg population is highly heterogeneous. Thus, the expression of many membrane and intracellular markers of these cells, including FOXP3 and CD25, varies significantly depending on a number of factors, including the functional state of the cells, tissue localization, the presence of pathology or cytokines in the environment [27].
The CD3, CD4, CD25, CD127, and FOXP3 markers are the main markers for the identification of human Treg cells. Staining on Ki67 and CD45RA provides additional information about the Treg activation status. Each of the markers of regulatory T lymphocytes has its own functional significance for the correct functioning of cells:
- The CD3 multiprotein complex is the main T cell receptor co-receptor, expressed on the membrane surface of all T lymphocyte subpopulations;
- Transmembrane glycoprotein of the CD4 immunoglobulins superfamily plays an αβ-TCRco-receptor role, participating in the recognition of antigen presented by antigen-presenting cells;
- CD25 protein is an alpha subunit of the low-affinity receptor for the anti-inflammatory cytokine IL-2, found on Treg, as well as on activated B cells, NK cells, myeloid progenitors, and oligodendrocytes;
- Transcription factor forkhead box protein P3, or scurfin (FOXP3), is a specific protein for activated CD4+CD25+ Stable expression of FOXP3 is necessary for the regulation of differentiation and functions of regulatory T lymphocytes. Defects in the FOXP3 gene lead to a deficiency or absence of normally functioning Tregs. However, FOXP3 is also important for the functioning of Treg in the tumor microenvironment [28];
- CD127 is an alpha chain of the IL-7 receptor. For regulatory T cells, its expression was found to be inversely proportional to the expression of FOXP3; therefore, CD127 is used as a negative Treg marker.
Among CD4+CD25+ lymphocytes, cells with a stable and unstable expression of the transcription factor FOXP3 are distinguished. At the same time, cells that do not express FOXP3 do not exhibit suppressive properties. It was noted [29] that a certain percentage of the total Treg population exhibit the capacity of the Treg/Tconv transformation, i.e., cells with suppressive effects and non-regulatory T-helper cells.
Role of regulatory T cells in the tumor microenvironment
Regulatory T lymphocytes, whose functioning is necessary to maintain an adequate immune response, are also an important component of the tumor microenvironment. Tregs exhibit significant plasticity and functional diversity in various tumors within the microenvironment [30].
Although the brain was considered an organ isolated from the peripheral immune system for a long time, now it is increasingly recognized as being involved in the structure of systemic immunity. The integration and interaction of the brain with the components of peripheral immunity require strict control and fine regulation. The key population providing additional mechanisms of immunoregulation in the brain are regulatory T lymphocytes [31]. However, in the case of malignant neoplasms, Tregs can contribute to the development of the tumor and its evasion from immune surveillance. In the late stages of the high-grade glioma development, damage to the blood-brain barrier often occurs, which additionally promotes the migration of Treg and other immune cells into the peri-tumoral space [32].
Tumors, including gliomas in particular, maintain a high level of immunosuppression in the microenvironment due to infiltration by regulatory cells. It was noted in [33] that in IDH-mutant glioma, the infiltration of TME Treg is less pronounced compared to the more aggressive IDH-wild type glioblastoma. A large amount of Treg accumulates in the tumor focus through selective chemokine-mediated recruitment of peripheral T lymphocytes. Tregs in patients with glioblastoma were shown to have a significantly higher level of CCR2 CCR4 receptor expression than Tregs in healthy people [34]. In addition to attracting peripheral regulatory T cells, tumors stimulate acquisition of a regulatory phenotype by naive CD4+ T cells [35]. It was found that the conditioned environment of glioblastoma can promote the expansion of Treg in vitro, which indicates the direct influence of factors produced by tumor cells on regulatory T lymphocytes [36].
Regulatory T lymphocytes exert an immunosuppressive effect in the tumor microenvironment due to several basic mechanisms (Fig. 1).
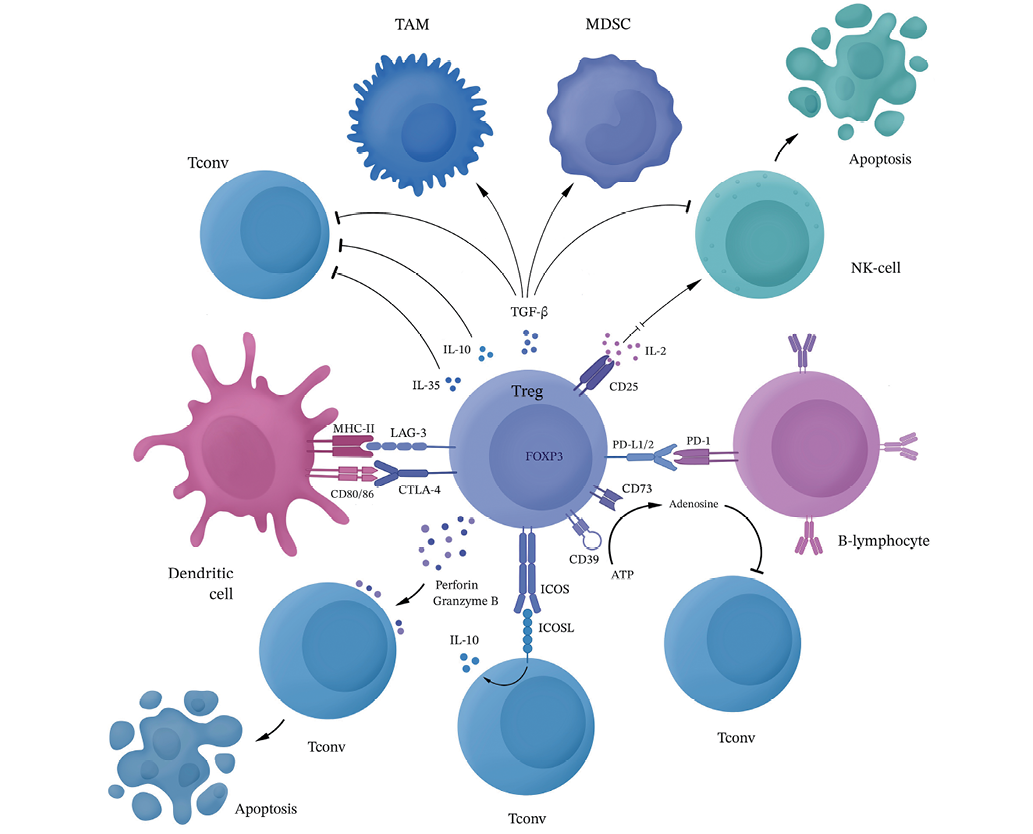
Figure prepared by the authors using data from the reference [37]
Fig. 1. Mechanisms of immunosuppressive action of regulatory T lymphocytes in the tumor microenvironment
Regulatory T cells produce granzyme B and perforin, acting on effector cells and stimulating their apoptosis.
- Tregs secrete TGF-β, IL-10, and IL-35 inhibitory cytokines, which inhibit CTL activity by binding to receptors on the surface of CD8+
- Cytotoxic T-lymphocytic protein 4 (CTLA-4) on the membrane of regulatory T lymphocytes competes with CD80/CD86 on the surface of T-killers, which leads to suppression of their activity and promotes the secretion of indolamine-2,3-dioxygenase (IDO); IDO activates signaling pathways of apoptosis of effector T cells. The interaction of CTLA-4 and LAG-3 with CD80/CD86 and MHC-II on the surface of dendritic cells also leads to suppression of their maturation and a decrease in the effective presentation of antigens.
- ICOS (inducible T cell costimulator) on the surface of Treg binds to ICOSL on the membrane of effector cells, stimulating the production of the anti-inflammatory IL-10 cytokine.
- The CD39/CD73 ectonucleotidase on the Treg membrane converts ATP into adenosine, which binds to CTL receptors and leads to a decrease in their functional activity.
Regulatory T cells ensure the escape of glioblastoma from antitumor immunity mainly by inhibiting CD8+ cytotoxic lymphocytes and reducing the functional activity of NK cells through intercellular interactions and secretion of soluble factors [38]. In addition, due to the production of TGF-β and IDO and a decrease in the secretion of IL-2 and IFN-γ, regulatory T lymphocytes can suppress antigen-presenting cells and increase the activity of TAM and MDSC, which contributes to the maintenance of the immunosuppressive microenvironment in the glioma focus. It was also noted that the FOXP3 transcription factor can induce the expression of heme oxygenase HO-1, which leads to the expansion and increased survival of the Treg population, as well as to a decrease in the expression of proinflammatory cytokines and suppression of the proliferation of effector T lymphocytes [39]. In addition, regulatory T cells can cause replicative aging and death of effector CD4+ T lymphocytes, CTL, B lymphocytes, and NK cells both in vitro and in vivo [40].
The TGF-β secreted by regulatory T lymphocytes is not only involved in the process of immunosuppression maintenance, but also acts on tumor cells by inducing the expression of the main genes associated with glioma stem cells (CD133, SOX2, NESTIN, MUSASHI1, and ALDH1A), as well as the NF-kB-IL6-STAT3 signaling pathway, which enhances the carcinogenic potential and glioblastoma stemming [41].
The multitude of mechanisms of the immunosuppressive action of regulatory T lymphocytes in the glioblastoma microenvironment can serve as the basis for the development of targeted drugs for certain metabolic pathways and Treg effects. However, such a diversity creates difficulties in selecting the necessary foci of action for therapy.
Targets of regulatory T lymphocytes for targeted therapy
Regulatory T lymphocytes make a significant contribution to tumor progression, invasion, and therapeutic resistance; therefore, they can act as a target for the treatment of patients with glioblastoma [42]. Currently, drugs aimed at various types of targets and metabolic processes of Treg are being developed and undergoing preclinical and clinical studies for targeted therapy [43].
Although systemic depletion of regulatory T cells can lead to increased antitumor immunity, this process is accompanied by severe autoimmune reactions. Therefore, numerous studies have attempted to selectively deplete regulatory T cells only in tumors, without affecting Tregs in healthy tissues. A decrease in the activity and proliferation of effector T cells (including due to exposure to Treg) leads to low effectiveness of, e.g., CAR-T therapy. At the same time, it was found that a combination with the therapy aimed at depletion of the total number of T cells increases the effectiveness of not only CAR-T [44], but also radioimmunotherapy treatment [45].
One possible approach to selectively affect Treg consists in the use of drugs targeting receptors for certain interleukins essential for the functioning of regulatory T lymphocytes. These include, e.g., drugs against the alpha chain of the IL-2 and CD25 receptors [46]. A member of the tumor necrosis factor (TNF) OX40 (CD134) receptor superfamily is mainly expressed by CD4+ and CD8+ T cells, while tumor-infiltrating Tregs exhibit a higher OX40 expression than peripheral Tregs. After TCR activation on TILs, OX40 is temporarily expressed to transmit a powerful costimulatory signal when it is bound to OX40L. Thus, OX40 agonists can enhance antitumor immunity [47]. The TNF CD27 receptor, as well as its CD70 ligand, can also act as a target [48].
The metabolic pathways of activation and inhibition of regulatory T cells, as well as transcription factors and various costimulating molecules can act as targets for targeted drugs [49]. Drugs of this type include, e.g., checkpoint inhibitors (CTLA-4, IDO, programmed cell death protein 1 — PD-1, T cell immunoglobulin 3 — Tim-3, STAT3 signaling pathway, etc.), which are successfully used in some malignancies [50]. The CTLA-4 receptor is constitutively expressed on naive Tregs and other T-lymphocyte populations; however, its expression is most pronounced in tumor-infiltrating Tregs. Monoclonal antibodies against CTLA-4 can deplete Treg cells in the tumor microenvironment through the mechanism of antibody-dependent cell-mediated cytotoxicity, thereby enhancing antitumor immunity [51]. Although ICB treatment (including the most widely used PD-1 blockers [52]) has not so far shown sufficient effectiveness in glioblastoma patients, some drugs of a new generation of inhibitors may be more effective [53].
The phenomenon of mutual transformation of activated Treg and unregulatory T lymphocytes not expressing FOXP3 (Treg/Tconv) can potentially be used for glioblastoma therapy. Indeed, shifting the balance in favor of inactive regulatory T lymphocytes may reduce the immunosuppression severity in the microenvironment, which in turn will lead to greater effectiveness of the patient’s own immune response and other types of therapy [54].
The current evidence shows that Tregs do not play such an unambiguous role in the tumor focus as previously thought. A number of studies confirm the antitumor activity of Treg and their correlation with improved prognosis in certain types of malignant neoplasms (stomach cancer, squamous cell carcinoma of the head and neck, colorectal cancer, etc.) [55]. Regulatory T cells, on the one hand, suppress inflammatory reactions that contribute to the progression of certain types of tumors; on the other, some Treg subpopulations can enhance antitumor immunity. For example, targeting the glucocorticoid-induced TNFR-related protein (GITR) of regulatory T cells with an agonist antibody (αGITR) promotes CD4+ differentiation Treg in effector T cells. Reprogrammed regulatory T lymphocytes express genes characteristic of Th1, produce IFN-γ, and acquire cytotoxic activity against glioma cells, while losing their suppressive function. In turn, αGITR and αPD1 combined with standard treatment of newly diagnosed glioblastoma increased recovery rates in experimental models [56].
In addition to affecting regulatory T lymphocytes directly, the attraction of Treg from peripheral blood into the glioblastoma microenvironment is also possible [57]. Modulation of the interaction of chemokines and their receptors can be used to develop immunotherapeutic drugs for the treatment of malignant gliomas.
CONCLUSION
Pronounced immunosuppression and a high cellular heterogeneity in the glioblastoma focus prevent the development of a natural antitumor response, thus reducing the effectiveness of standard treatment methods.
Regulatory T lymphocytes play an ambiguous role. On the one hand, Tregs are necessary for maintaining immune homeostasis in the body. On the other, regulatory T cells in the glioblastoma microenvironment ensure the escape of the tumor from immunological surveillance. Due to intercellular contacts and secretion of anti-inflammatory cytokines, perforins, granzymes, and other biologically active molecules, regulatory T lymphocytes suppress the activity and proliferation of effector cells in the microenvironment, contributing to the growth and progression of glioblastoma.
Currently, the development of effective and highly selective therapy for malignant gliomas remains an urgent task for researchers. Trials of new therapeutic drugs and modified treatment regimens are necessary to improve the quality of life and the overall survival of patients with glioblastoma, while reducing the incidence of side effects and disease relapses. Regulatory T cells make a significant contribution to the suppression of antitumor immunity and can act as a target for cancer therapy. To reduce the activity and total number of Tregs, drugs acting on interleukin receptors, chemokines, costimulating molecules, metabolic pathways of regulatory T cells, etc., can be used.
In addition to the treatment aimed at regulatory T lymphocytes, other approaches are currently undergoing preclinical and clinical trials: CAR-T therapy, dendritic vaccines, microRNAs, mRNAs, oncolytic virus therapy, etc. It is worth noting that the effectiveness of treatment of glioblastoma patients (in particular, immunotherapy and oncolytic virus therapy) largely depends on the level of local and systemic suppression of the immune response, which requires attention when selecting personalized therapeutical approaches.
An improved understanding of the functional diversity and metabolic features of regulatory T lymphocytes as a key component of tumor immunosuppression, as well as the study of their interaction with other cells in the microenvironment, may offer new possibilities for the treatment of malignant gliomas. In the future, the use of various therapeutic methods as part of a combination treatment, including with targeted Treg drugs, may demonstrate greater effectiveness in comparison with standard approaches.
References
1. Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, Mampre D, Jackson C, Peterson J, et al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147:297–307. https://doi.org/10.1007/s11060-020-03451-6
2. Lah TT, Novak M, Breznik B. Brain malignancies: Glioblastoma and brain metastases. Seminars in Cancer Biology. 2020;60:262–73. https://doi.org/10.1016/j.semcancer.2019.10.010
3. Smith CJ, Perfetti TA, Chokshi C, Venugopal C, Ashford JW, Singh SK. Risk factors for glioblastoma are shared by other brain tumor types. Hum Exp Toxicol. 2024;43:9603271241241796. https://doi.org/10.1177/09603271241241796
4. Colopi A, Fuda S, Santi S, Onorato A, Cesarini V, Salvati M, et al. Impact of age and gender on glioblastoma onset, progression, and management. Mech Ageing Dev. 2023;211:111801. https://doi.org/10.1016/j.mad.2023.111801
5. Khan I, Mahfooz S, Elbasan EB, Karacam B, Oztanir MN, Hatiboglu MA. Targeting Glioblastoma: The Current State of Different Therapeutic Approaches. Curr Neuropharmacol. 2021;19:1701–15. https://doi.org/10.2174/1570159X19666210113152108
6. Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, et al. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacological Research. 2021;171:105780. https://doi.org/10.1016/j.phrs.2021.105780
7. Wang X, Ge Y, Hou Y, Wang X, Yan Z, Li Y, et al. Single-cell atlas reveals the immunosuppressive microenvironment and Treg cells landscapes in recurrent Glioblastoma. Cancer Gene Ther. 2024;31:790–801. https://doi.org/10.1038/s41417-024-00740-4
8. Lin H, Liu C, Hu A, Zhang D, Yang H, Mao Y. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J Hematol Oncol. 2024;17:31. https://doi.org/10.1186/s13045-024-01544-7
9. Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151:41–53. https://doi.org/10.1007/s11060-020-03448-1
10. Bikfalvi A, da Costa CA, Avril T, Barnier J-V, Bauchet L, Brisson L, et al. Challenges in glioblastoma research: focus on the tumor microenvironment. Trends Cancer. 2023;9:9–27. https://doi.org/10.1016/j.trecan.2022.09.005
11. Bugakova AS, Chudakova DA, Myzina MS, Yanysheva EP, Ozerskaya IV, Soboleva AV, et al. Non-Tumor Cells within the Tumor Microenvironment — The «Eminence Grise» of the Glioblastoma Pathogenesis and Potential Targets for Therapy. Cells. 2024;13. https://doi.org/10.3390/cells13100808
12. Dinevska M, Widodo SS, Furst L, Cuzcano L, Fang Y, Mangiola S, et al. Cell signaling activation and extracellular matrix remodeling underpin glioma tumor microenvironment heterogeneity and organization. Cell Oncol (Dordr). 2023;46:589–602. https://doi.org/10.1007/s13402-022-00763-9
13. Qiu R, Zhong Y, Li Q, Li Y, Fan H. Metabolic Remodeling in Glioma Immune Microenvironment: Intercellular Interactions Distinct From Peripheral Tumors. Front Cell Dev Biol. 2021;9:693215. https://doi.org/10.3389/fcell.2021.693215
14. Pereira MB, Barros LRC, Bracco PA, Vigo A, Boroni M, Bonamino MH, et al. Transcriptional characterization of immunological infiltrates and their relation with glioblastoma patients overall survival. Oncoimmunology. 2018;7:1431083. https://doi.org/10.1080/2162402X.2018.1431083
15. Stepanenko AA, Sosnovtseva AO, Valikhov MP, Chernysheva AA, Abramova OV, Pavlov KA, et al. Systemic and local immunosuppression in glioblastoma and its prognostic significance. Front Immunol. 2024;15. https://doi.org/10.3389/fimmu.2024.1326753
16. Dapash M, Hou D, Castro B, Lee-Chang C, Lesniak MS. The Interplay between Glioblastoma and Its Microenvironment. Cells. 2021;10:2257. https://doi.org/10.3390/cells10092257
17. Himes BT, Geiger PA, Ayasoufi K, Bhargav AG, Brown DA, Parney IF. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front Oncol. 2021;11:770561. https://doi.org/10.3389/fonc.2021.770561
18. Du M, Sun L, Guo J, Lv H. Macrophages and tumor-associated macrophages in the senescent microenvironment: From immunosuppressive TME to targeted tumor therapy. Pharmacological Research. 2024;204:107198. https://doi.org/10.1016/j.phrs.2024.107198
19. Mi Y, Guo N, Luan J, Cheng J, Hu Z, Jiang P, et al. The Emerging Role of Myeloid-Derived Suppressor Cells in the Glioma Immune Suppressive Microenvironment. Front Immunol. 2020;11. https://doi.org/10.3389/fimmu.2020.00737
20. Vilbois S, Xu Y, Ho P-C. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer. 2024;10:242–55. https://doi.org/10.1016/j.trecan.2023.11.007
21. Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117–40. https://doi.org/10.1007/978-3-030-35723-8_8
22. Iglesias-Escudero M, Arias-González N, Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Molecular Cancer. 2023;22:26. https://doi.org/10.1186/s12943-023-01714-0
23. Shang J, Zha H, Sun Y. Phenotypes, Functions, and Clinical Relevance of Regulatory B Cells in Cancer. Front Immunol. 2020;11:582657. https://doi.org/10.3389/fimmu.2020.582657
24. Zwirner NW, Domaica CI, Fuertes MB. Regulatory functions of NK cells during infections and cancer. Journal of Leukocyte Biology. 2021;109:185–94. https://doi.org/10.1002/JLB.3MR0820-685R
25. Zhang Z, Guo J, Jia R. Treg plasticity and human diseases. Inflamm Res. 2023;72:2181–97. https://doi.org/10.1007/s00011-023-01808-x
26. Churov AV, Novitskaya AV, Zhulay GA. Immunoterapiya novogo pokoleniya: regulyatornye T-kletki. Genes & cells. 2021;16(3);16–32. (In Russ.). https://doi.org/10.23868/202110003
27. Blinova VG, Zhdanov DD. Many Faces of Regulatory T Cells: Heterogeneity or Plasticity? Cells. 2024;13:959. https://doi.org/10.3390/cells13110959
28. Wang J, Gong R, Zhao C, Lei K, Sun X, Ren H. Human FOXP3 and tumour microenvironment. Immunology. 2023;168:248–55. https://doi.org/10.1111/imm.13520
29. Sadlon T, Brown CY, Bandara V, Hope CM, Schjenken JE, Pederson SM, et al. Unravelling the molecular basis for regulatory T-cell plasticity and loss of function in disease. Clin Transl Immunology. 2018;7(2):1011. https://doi.org/10.1002/cti2.1011
30. Glasner A, Plitas G. Tumor resident regulatory T cells. Semin Immunol. 2021;52:101476 https://doi.org/10.1016/j.smim.2021.101476
31. Liston A, Pasciuto E, Fitzgerald DC, Yshii L. Brain regulatory T cells. Nat Rev Immunol. 2024;24:326–37. https://doi.org/10.1038/s41577-023-00960-z
32. Ratnam NM, Gilbert MR, Giles AJ. Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro-Oncology. 2019;21:37–46. https://doi.org/10.1093/neuonc/noy084
33. Richardson LG, Nieman LT, Stemmer-Rachamimov AO, Zheng XS, Stafford K, Nagashima H, et al. IDH-mutant gliomas harbor fewer regulatory T cells in humans and mice. Oncoimmunology. 2020;9:1806662. https://doi.org/10.1080/2162402X.2020.1806662
34. Panek WK, Toedebusch RG, Mclaughlin BE, Dickinson PJ, Van Dyke JE, Woolard KD, et al. The CCL2-CCR4 axis promotes Regulatory T cell trafficking to canine glioma tissues. J Neurooncol. 2024;169:647–58. https://doi.org/10.1007/s11060-024-04766-4
35. Canella A, Artomov M, Ukhatov A, Rajendran S, Perez P, Saini U, et al. Primary murine high-grade glioma cells derived from RCAS/tv-a diffuse glioma model reprogram naive T cells into immunosuppressive regulatory T lymphocytes. Mol Ther Oncol. 2024;32:200861. https://doi.org/10.1016/j.omton.2024.200861
36. Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro-Oncology. 2012;14:5:584–95. https://doi.org/10.1093/neuonc/nos014
37. Dikiy S, Rudensky AY. Principles of regulatory T cell function. Immunity. 2023;56:240–55. https://doi.org/10.1016/j.immuni.2023.01.004
38. Lu L, Sun J, Su H, Luo S, Chen J, Qiu S, et al. Antitumor CD8 T cell responses in glioma patients are effectively suppressed by T follicular regulatory cells. Exp Cell Res. 2021;407:112808. https://doi.org/10.1016/j.yexcr.2021.112808
39. Luu Hoang KN, Anstee JE, Arnold JN. The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front Immunol. 2021;12. https://doi.org/10.3389/fimmu.2021.658315
40. Zhdanov DD, Gladilina YA, Pokrovsky VS, Grishin DV, Grachev VA, Orlova VS, et al. Murine regulatory T cells induce death of effector T, B, and NK lymphocytes through a contact-independent mechanism involving telomerase suppression and telomereassociated senescence. Cell Immunol. 2018;331:146–60. https://doi.org/10.1016/j.cellimm.2018.06.008
41. Liu S, Zhang C, Wang B, Zhang H, Qin G, Li C, et al. Regulatory T cells promote glioma cell stemness through TGF-β–NF-κB–IL6–STAT3 signaling. Cancer Immunol Immunother. 2021;70:2601–16. https://doi.org/10.1007/s00262-021-02872-0
42. Wang Y, Huang T, Gu J, Lu L. Targeting the metabolism of tumor-infiltrating regulatory T cells. Trends in Immunology. 2023;44:598–612. https://doi.org/10.1016/j.it.2023.06.001
43. Chen B-J, Zhao J-W, Zhang D-H, Zheng A-H, Wu G-Q. Immunotherapy of Cancer by Targeting Regulatory T cells. Int Immunopharmacol. 2022;104:108469. https://doi.org/10.1016/j.intimp.2021.108469
44. Wang AX, Ong XJ, D’Souza C, Neeson PJ, Zhu JJ. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front Immunol. 2023;14. https://doi.org/10.3389/fimmu.2023.1140541
45. van Hooren L, Handgraaf SM, Kloosterman DJ, Karimi E, van Mil LWHG, Gassama AA, et al. CD103 + regulatory T cells underlie resistance to radio-immunotherapy and impair CD8 + T cell activation in glioblastoma. Nat Cancer. 2023;4:665–81. https://doi.org/10.1038/s43018-023-00547-6
46. Peng Y, Tao Y, Zhang Y, Wang J, Yang J, Wang Y. CD25: A potential tumor therapeutic target. Int J Cancer. 2023;152:1290–303. https://doi.org/10.1002/ijc.34281
47. Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood. 2018;131:39–48. https://doi.org/10.1182/blood-2017-07-741025
48. Muth S, Klaric A, Radsak M, Schild H, Probst HC. CD27 expression on Treg cells limits immune responses against tumors. J Mol Med (Berl). 2022;100:439–49. https://doi.org/10.1007/s00109-021-02116-9
49. Li Q, Lu J, Li J, Zhang B, Wu Y, Ying T. Antibody-based cancer immunotherapy by targeting regulatory T cells. Front Oncol. 2023;13:1157345. https://doi.org/10.3389/fonc.2023.1157345
50. Zhulai G, Oleinik E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand J Immunol. 2022;95:13129. https://doi.org/10.1111/sji.13129
51. Arrieta VA, Dmello C, McGrail DJ, Brat DJ, Lee-Chang C, Heimberger AB, et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. The Journal of Clinical Investigation. 2023;133:163447. https://doi.org/10.1172/JCI163447
52. Yang T, Kong Z, Ma W. PD-1/PD-L1 immune checkpoint inhibitors in glioblastoma: clinical studies, challenges and potential. Hum Vaccin Immunother. 2021;17:546–53. https://doi.org/10.1080/21645515.2020.1782692
53. Badani A, Ozair A, Khasraw M, Woodworth GF, Tiwari P, Ahluwalia MS, et al. Immune checkpoint inhibitors for glioblastoma: emerging science, clinical advances, and future directions. J Neurooncol. 2025;171:531–47. https://doi.org/10.1007/s11060-024-04881-2
54. Whiteside SK, Grant FM, Alvisi G, Clarke J, Tang L, Imianowski CJ, et al. Acquisition of suppressive function by conventional T cells limits anti-tumor immunity upon Treg depletion. Sci Immunol. 2023;8:5558. https://doi.org/10.1126/sciimmunol.abo5558
55. Li Y, Zhang C, Jiang A, Lin A, Liu Z, Cheng X, et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J Transl Med. 2024;22:293. https://doi.org/10.1186/s12967-024-05104-y
56. Amoozgar Z, Kloepper J, Ren J, Tay RE, Kazer SW, Kiner E, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun. 2021;12:2582. https://doi.org/10.1038/s41467-021-22885-8
57. Panek WK, Pituch KC, Miska J, Kim JW, Rashidi A, Kanojia D, et al. Local Application of Autologous Platelet-Rich Fibrin Patch (PRF-P) Suppresses Regulatory T Cell Recruitment in a Murine Glioma Model. Mol Neurobiol. 2019;56:5032–40. https://doi.org/10.1007/s12035-018-1430-0
About the Authors
E. P. YanyshevaRussian Federation
Elvira P. Yanysheva
Moscow
V. P. Baklaushev
Russian Federation
Vladimir P. Baklaushev, Dr. Sci. (Med.)
Moscow
G. M. Yusubalieva
Russian Federation
Gaukhar M. Yusubalieva, Cand. Sci. (Med.)
Moscow
Supplementary files
Review
For citations:
Yanysheva E.P., Baklaushev V.P., Yusubalieva G.M. Role of regulatory T lymphocytes in the formation of immunosuppressive microenvironment in glioblastoma. Extreme Medicine. 2025;27(2):183-190. https://doi.org/10.47183/mes.2025-299

















