Scroll to:
In vitro evaluation of Acinetobacter baumannii resistance to tigecycline in Iran: a systematic review and meta-analysis
https://doi.org/10.47183/mes.2025-318
Abstract
Introduction. Acinetobacter baumannii (A. baumannii) is a widespread and exclusively hospital-acquired microorganism whose new mutation is resistant to most available antibiotics, with the exception of tigecycline. Clinicians are concerned about recent evidence of resistance to this antibiotic in Iran.
Objective. This study evaluates the resistance of Acinetobacter baumannii (A. baumannii) to tigecycline in Iran, considering its clinical significance in treating multi-drug-resistant infections.
Methods. The MEDLINE, PubMed, Web of Science (WOS), and Scopus databases were searched for studies published over all this time to January 2024. The advanced search using Medical Subject Headings (MeSH) terms for “Acinetobacter baumannii” and “Tigecycline” was performed. The title, abstract, and full text of the articles were screened based on eligibility criteria. The cross-sectional studies reporting Tigecycline resistance in sequential isolates of A. baumannii in patients admitted to the hospitals in Iran were included.
Results. A total of 16 studies were included for meta-analysis. The overall prevalence of A. baumannii strains resistant to tigecycline in Iran equals 18.1%. Among the reviewed studies, distinct variances of resistance were detected. Although investigations were conducted in limited regions, the studies reported a wide range of resistance in Tehran (0%) and in Tabriz (100%) as minimum and maximum, respectively.
Conclusion. Despite the high level of resistance in some cities of Iran, tigecycline is still one of the most effective antibiotics for the treatment of A. baumannii infection. Improved control over the use of antibiotics may contribute to hampering the spread of resistance to these agents.
Keywords
For citations:
Rahmanian M., Varjovi M.N., Deravi N., Nariman Z., Gholamzad A., Keylani K., Alizadeh A., Mousavianfard S.R. In vitro evaluation of Acinetobacter baumannii resistance to tigecycline in Iran: a systematic review and meta-analysis. Extreme Medicine. 2025;27(2):257-266. https://doi.org/10.47183/mes.2025-318
INTRODUCTION
Acinetobacter baumannii (A. baumannii) is a ubiquitous, aerobic, gram-negative coccobacillus. This bacterium is widely spread in water, soil, and hospital environments, capable of surviving there for a long time [1][2]. In addition, A. baumannii is a common pathogen identified in the blood, skin, urine, pleural fluid, and sputum [3]. Due to its capacity to transfer between non-living and living objects, this pathogen is increasingly responsible for hospital-acquired infections [4]. Moreover, the emergence, proliferation, and spread of a new drug-resistant A. baumannii, which is capable of transferring some genetic elements and is resistant to different antibiotics, has worsened the situation [5]. Various studies have reported the resistance of A. baumannii to different classes of antimicrobial agents, such as aminoglycosides, β-lactams, and quinolones, as well as carbapenems. Given the scarcity of alternatives available for treating drug-resistant infections caused by A. baumannii, tigecycline is currently attracting research attention [2][6].
In comparison with other tetracyclines, tigecycline is a bacteriostatic agent with a higher binding affinity to the bacterial 30S ribosomal subunit. Tigecycline, an antibiotic based on minocycline, has a wide spectrum of action and is capable of overcoming the main mechanisms of bacterial resistance to tetracyclines, such as efflux and ribosome protection. This is achieved by adding a glycylamide fragment to the minocycline molecule [7]. The mechanism of resistance to tetracyclines is generally mediated by the following systems: the attainment of genetic sections transferring the genes particularly resistant to tetracyclines, mutations inside the attaching region of the ribosome, and/or mutations in chromosomes leading to intensified expression of fundamental resistance mechanisms. Various processes of bacterial resistance were described in [8].
Tigecycline is a semisynthetic agent known as the primary exclusive antibiotic of the glycylamide class. Tigecycline overcomes key tetracycline resistance mechanisms — namely, efflux pump activity and ribosomal protection — through the addition of a glycyclamide group to its minocycline-based structure. This structural modification contributes to its broad-spectrum antibacterial activity [7]. Tigecycline is an available drug for treating multidrug-resistant A. baumannii [9], showing activity against multiple drug resistant (MDR) pathogens such as Enterobacteriaceae, Staphylococcus aureus, and Acinetobacter species [10]. In 2005, the US Food and Drug Administration (US FDA) approved this drug for treating community-acquired pneumonia, skin infections (except for diabetes foot infection), and complicated intra-abdominal infections [11].
Tigecycline exhibits a noticeable activity against extensive drug resistance (XDR) and MDR gram-negative bacteria, particularly A. baumannii. The resistance of the latter to tigecycline has been reported relatively recently [12–14]. Some mechanisms, such as the chromosomal or supplemental encoding process of genes, are responsible for tigecycline resistance [7]. Since tigecycline is one of the few remaining drugs for the treatment of A. baumannii infection with MDR, it is necessary to determine its potential resistance in a timely manner. In comparison with developed countries, where effective preventive health measures are used, developing countries, such as Iran, require the development and implementation of measures to control the process of drug prescription, disseminate information about antibiotic resistance among patients, and regulate the proper use of medicines [15]. Thus, the study of A. baumannii resistance to tigecycline in Iranian patients is an important and urgent task.
The purpose of this study was to evaluate the resistance of Acinetobacter baumannii (A. baumannii) to tigecycline in Iran, taking into account its clinical significance in the treatment of multidrug-resistant infections.
MATERIALS AND METHODS
This research was designed in accordance with Cochrane’s standard methodology and presented based on the PRISMA checklist (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
Search strategy
The literature search was conducted across the Google Scholar, PubMed, Web of Science, and Scopus databases, covering all available data up to January 2024. The search algorithm based on keywords, their synonyms, and related Medical Subject Headings (MeSH terms) was as follows: ((Acinetobacter baumannii [title/abstract]) OR (Acinetobacter [Title/Abstract])) OR (A. baumannii [Title/Abstract]) AND (Tygacil [Title/Abstract])) OR ((Tigecycline [title/abstract]). In addition, a backward and forward citation search was conducted to raise the comprehensiveness of the conducted literature search.
Inclusion and exclusion criteria
The records achieved from searching the databases were combined in the EndNote X9 library (Thomson Reuters, Toronto, ON, Canada); duplicates were deleted.
The resulting sample included in vitro cross-sectional studies investigating the resistance of A. baumannii to tigecycline in sequential A. baumannii isolates from the patients admitted to Iranian hospitals by using different methods, such as broth microdilution, disk diffusion, and E-test [16]. Moreover, only patients with MDR resistance were eligible for this study. Multidrug resistance is defined as not being susceptible to at least one antibiotic in three antimicrobial classes acknowledged as treatment options for the disease associated with A. baumannii [17]. Biological and biomedical research studies on animal models, as well as case reports, case series, case-controls, and studies evaluating variables irrelevant to resistance rate, were excluded.
Statistical analysis
The statistical analysis was conducted using the Comprehensive Meta-Analysis software package, version 3.0 (Biostat Inc., Englewood, NJ, USA). 95% confidence intervals (CIs) and point estimates for the resistance rate to tigecycline were calculated.
The degree of existing heterogeneity between different meta-studies was assessed using the I2 value and the p-criterion. The I2 value is a quantitative indicator of heterogeneity that shows a degree of inconsistency in research results. I2 describes the percentage of total variation between studies, which is due to heterogeneity rather than randomness. The I2 indicator is calculated based on the basic results obtained as a result of a typical meta-analysis, as
I2 = 100% × (Q – df) / Q, (1)
where Q is the Cochran heterogeneity statistic and df is the degree of freedom.
Negative I2 values were equated to zero such that I2 were ranging from 0 to 100%. A 0% value indicates the absence of heterogeneity, with higher values indicating an increase in heterogeneity. I2 statistics and Cochran’s Q-test were used to assess the heterogeneity between studies. Due to the high level of heterogeneity between studies (I2 > 50% or p < 0.1), a random effect model was used. To assess the reliability of the publication, we used the Egger criterion. Accordingly, the value of p < 0.05 was considered a statistically significant indicator for the reliability of the publication.
RESULTS
Study selection
The conducted systematic search across scientific databases produced 365 relevant articles that evaluate the prevalence of tigecycline-resistant A. baumannii in Iran. The first screening identified 190 articles by title and abstract, while the second screening based on full text found 53 articles. Following application of the inclusion and exclusion criteria, 16 articles were deemed satisfactory, thus being included in the current systematic review and meta-analysis (Figure 1).
Characteristics of the included studies
All selected articles reported cross-sectional studies conducted from 2014 to 2022 and spanning different geographical domains—capital (n = 10), north (n = 2), south (n = 3), and southwest (n = 1) — with various types of samples, including burn wound, urine, sputum, blood, and other body fluids (Table 1).
The quantity of MDR isolates ranged from 26 to 200. Nine, four, three, and one studies used disk diffusion, broth microdilution, E-test methods, and a combination of disk diffusion and broth microdilution methods for antimicrobial susceptibility testing on A. baumannii, respectively (Table 2).
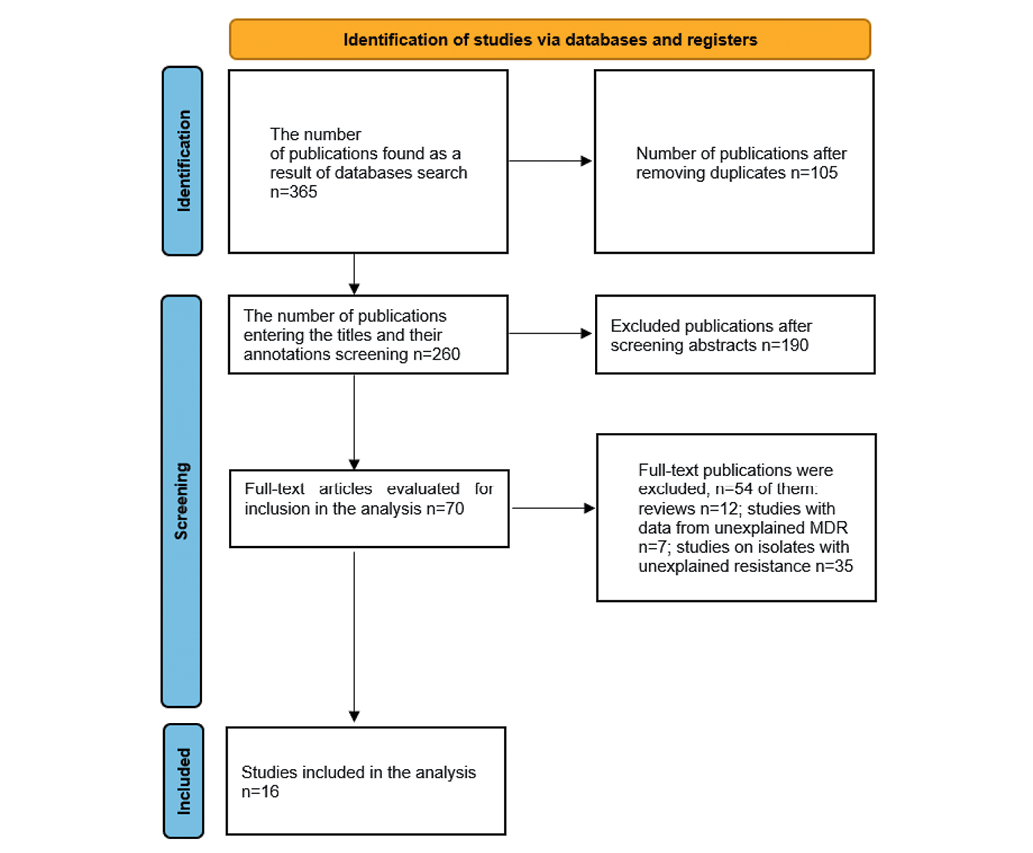
Figure prepared by the authors
Fig. 1. Flowchart diagram of the literature search procedure
Table 1. Characteristics of the included studies
|
Study |
Published year |
Year of study |
Type of study |
City of study |
Study population |
|
|
1 |
Sepahvand et al. [18] |
2022 |
no data |
Cross-Sectional |
Shiraz |
hospital patients |
|
2 |
Saadati et al. [13] |
2021 |
August 2017 to February 2018 |
Cross-Sectional |
Tabriz |
hospital patients |
|
3 |
Alavi-Moghaddam et al. [19] |
2020 |
January 2016 to November 2018 |
Cross-Sectional |
Tehran |
hospital patients |
|
4 |
Salehi et al. 2019 [20] |
2019 |
August 2016 and February 2017 |
Cross-Sectional |
Tehran |
hospital patients |
|
5 |
Tafreshi et al. [21] |
2019 |
between 2016 and 2018 |
Cross-Sectional |
Tehran |
hospital patients |
|
6 |
Yazdansetad et al. [22] |
2019 |
during 2013 |
Cross-Sectional |
Tehran |
hospital burned patients |
|
7 |
Salehi et al. 2018 [23] |
2018 |
no data |
Cross-Sectional |
Tehran |
patients, staff, and environment of an educational hospital |
|
8 |
Zafari et al. [24] |
2017 |
September 2015 to June 2016 |
Cross-Sectional |
Tehran |
hospital patients |
|
9 |
Sarhaddi et al. [25] |
2017 |
January and December 2014 |
Cross-Sectional |
Mashhad |
hospital patients |
|
10 |
Ansari et al. [26] |
2017 |
September 2015 to April 2016 |
Cross-Sectional |
Shahrekord |
hospital patients |
|
11 |
Alaei et al. [27] |
2016 |
February 2010 and March 2011 |
Cross-Sectional |
Shiraz |
ICU patients |
|
12 |
Pourhajibagher et al. [28] |
2016 |
no data |
Cross-Sectional |
Tehran |
hospital patients |
|
13 |
Jasemi et al. [29] |
2016 |
August 2011 to December 2013 |
Cross-Sectional |
Tehran |
hospital patients |
|
14 |
Kooti et al. [30] |
2015 |
December 2012 to May 2013 |
Cross-Sectional |
Shiraz |
hospital patients |
|
15 |
Bahador et al. 2015 [31] |
2015 |
2012 |
Cross-Sectional |
Tehran |
Burned patients |
|
16 |
Bahador et al. 2014 [32] |
2014 |
2011 |
Cross-Sectional |
Tehran |
ICU patients |
|
17 |
Bahador et al. 2014 [32] |
2014 |
2006 |
Cross-Sectional |
Tehran |
ICU patients |
Table prepared by the authors using data from the included studies [13][18–32]
Table 2. List of selected samples and research methods
|
Study |
Sample source |
MDR isolates |
TGC /MDR |
Diagnostic test |
|
|
1 |
Sepahvand et al [18] |
blood, wound, respiratory and urine samples |
100 |
22 |
disc diffusion |
|
2 |
Saadati et al [13] |
tracheal secretion, blood, wound, catheter, bronchial washing, CSF, urine, sputum, and ascites fluid |
100 |
100 |
disk diffusion |
|
3 |
Alavi-Moghaddam et al [19] |
blood, trachea, urine, cerebrospinal fluid, catheter and pleural fluid |
109 |
35 |
disc diffusion |
|
4 |
Salehi et al 2019 [20] |
various specimens mostly sputum |
180 |
152 |
disk diffusion |
|
5 |
Tafreshi et al [21] |
burn wound infection |
84 |
28 |
broth microdilution |
|
6 |
Yazdansetad et al [22] |
burn wound |
63 |
22 |
broth microdilution |
|
7 |
Salehi et al 2018 [23] |
wet swab from clothes and hands of staff, medical equipment, and patients’ environment |
125 |
2 |
disk diffusion |
|
8 |
Zafari et al [24] |
blood, wound, urine, sputum, and respiratory tract |
100 |
2 |
disc diffusion |
|
9 |
Sarhaddi et al [25] |
burnt wound |
54 |
2 |
E-test |
|
10 |
Ansari et al [26] |
clinical samples |
30 |
18 |
disk diffusion |
|
11 |
Alaei et al [27] |
urine, sputum, blood, postoperative wound, cerebrospinal fluid, nasal secretion, eye secretion |
45 |
4 |
broth microdilution |
|
12 |
Pourhajibagher et al [28] |
burn wound |
33 |
2 |
disk diffusion and broth microdilution |
|
13 |
Jasemi et al [29] |
clinical specimens |
26 |
8 |
disk diffusion |
|
14 |
Kooti et al [30] |
urine, wound, blood, sputum, ETT, body fluid, nose, throat and eye |
200 |
4 |
disk diffusion |
|
15 |
Bahador et al 2015 [31] |
clinical samples |
62 |
11 |
broth microdilution |
|
16 |
Bahador et al 2014 [32] |
wound, respiratory tract, urine, blood, and CSF |
50 |
4 |
E-Test |
|
17 |
Bahador et al 2014 [32] |
wound, respiratory tract, urine, blood, and CSF |
50 |
0 |
E-Test |
Table prepared by the authors using data from the included studies [13][18–32]
Note: TGC — Tigecycline; CSF — сerebrospinal fluid, ETT — endotracheal tube.
Prevalence and Genetic Mechanisms of Tigecycline Resistance in A. baumannii
The studies under analysis reported differing data on the resistance prevalence of A. baumannii. Thus, although Saadati et al. [13] found all of the isolates to be MDR (100 out of 100) and reported the highest resistance rate (100%) against tigecycline in the northwest of Iran, Salehi et al. [33] identified 1.6% of MDR clinical isolates (2 out of 125) resistant to tigecycline in the North of Iran. Bahador et al. [31] conducted a five-year-long study in the north of Iran and found a notable rise in resistance to tigecycline, with all isolates demonstrating susceptibility in 2006. However, by 2011, 8% of isolates had exhibited resistance. In contrast to southern regions, a study by Kooti et al. conducted in 2015 [30] detected resistance to tigecycline in 2% (4 out of 200) of MDR isolates. However, in 2016, Alaei et al. [27] reported an estimated resistance rate of 8.8% (4 out of 45) within the same region. In investigations conducted within the capital of Iran from 2016 to 2018, a wide scattering of results spanning from 1.6 to 84% was observed. Jasemi et al. [29] carried out multi-center research to evaluate the prevalence and trajectory of drug-resistant A. baumannii phenotypes from 2011 to 2013 in Tehran. Eventually, the researchers observed a remarkable decrease in the resistance of this pathogen to tigecycline.
The resistance mechanism to this antibiotic refers to the genotypic profiles of multidrug-resistant Acinetobacter baumannii (MDR-AB) isolates and involves genes in the resistance process.
In the study by Kooti et al. [30], 0.5, 7, and 40% of isolates possessed blaOXA-58-like, blaOXA-24-like, and blaOXA-23-like (class D beta-lactamase family) genes, respectively, using multiplex-polymerase chain reaction (PCR). In another study, by Bahador et al. [31], ISAba1 and ISAba4 (transposase family) were identified upstream of blaOXA-23-like genes in 45.1% and 12.9% of isolates, respectively. Moreover, Sarhaddi et al. [25] estimated the occurrence rates of blaTEM (class A beta-lactamase family), blaOXA-23-like, blaOXA-24-like, blaVIM, and blaIMP (subclass B1 metallo-beta-lactamase family) at the level of 64.8, 66.7, 68.5, 70.4, and 70.4%, respectively. The presence of these genes might demonstrate a correlation with the acquisition of antimicrobial resistance.
The forest plot presented in Fig. 2 based on the meta-analysis results, demonstrates an 18.1% antimicrobial resistance to tigecycline among MDR A. baumannii species. Concerning the heterogeneity test, the obtained results showed Q-value = 305.712, df (Q) = 16, p-value = 0.000, and I2 = 94.766 for the selected studies. The included studies demonstrated a publication bias, based on significant results of Egger’s test (p-value < 0.05) and the asymmetrical funnel plot (Fig. 3).
Subgroup analysis
The overall resistance rate in ICU and non-ICU was 0.072 and 0.222, respectively (Fig. 4). The ICU subgroup had a lower heterogeneity (I2 = 14.18%), while non-ICU patients showed a high heterogenicity (I2 = 95.28%).
The overall resistance rate in Shiraz and Tehran was 0.079 and 0.161, respectively (Fig. 5). I2 for these analyses was 91.31% for Shiraz and 95.14% for Tehran.
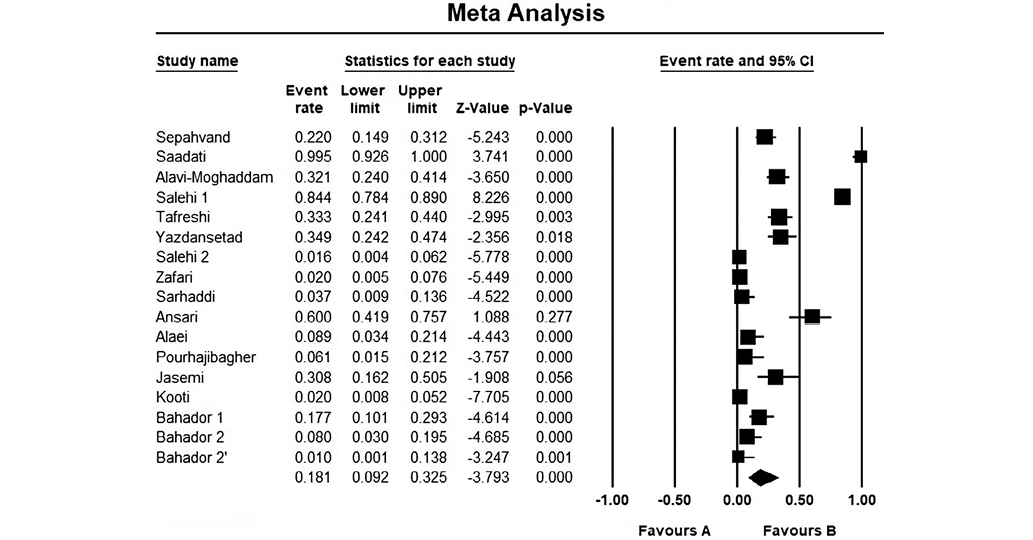
Figure prepared by the authors
Fig. 2. The results of the meta-analysis evaluating the resistance of different isolates of A. baumannii to tigecycline in Iran
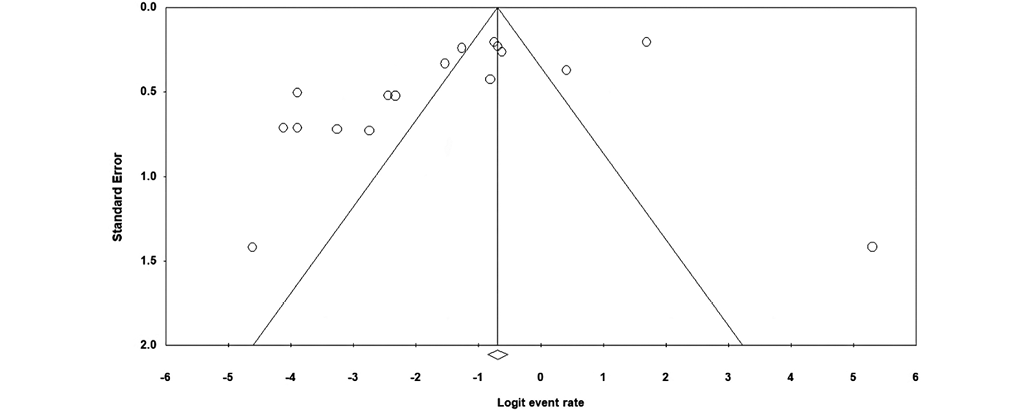
Figure prepared by the authors
Fig. 3. Funnel plot
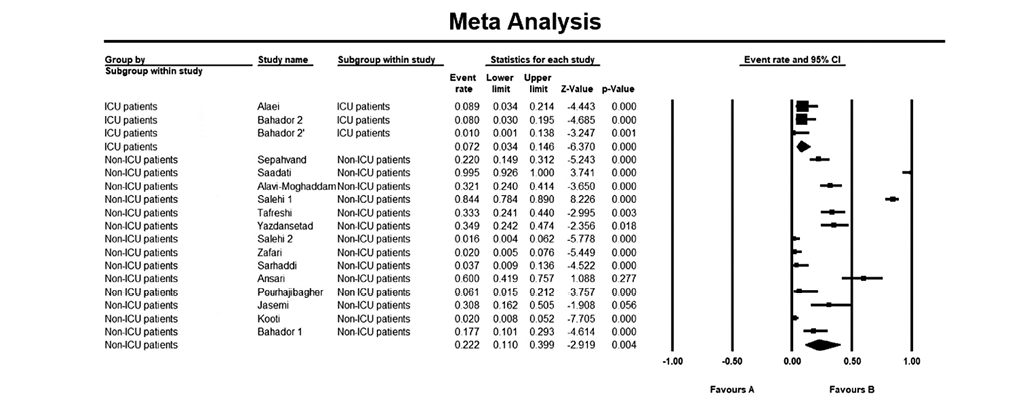
Figure prepared by the authors
Fig. 4. Subgroup analysis of patients admitted to ICU and non-ICU wards
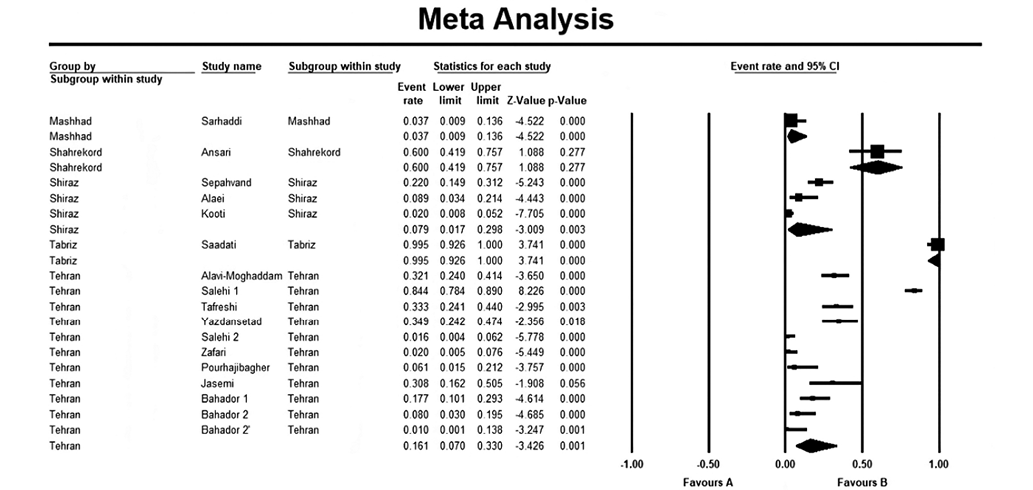
Figure prepared by the authors
Fig. 5. Subgroup analysis based on location
DISCUSSION
A. baumannii is a key agent of hospital-acquired infections due to its resistance to various classes of antibiotics [34][35]. For this reason, the use of effective antibiotics, and the ongoing monitoring of antimicrobial resistance may contribute to A. baumanniieradication. Tigecycline, a potent semi-synthetic derivative of tetracycline, is recognized as the primary option among novel pharmaceuticals for infections caused by MDR strains of Acinetobacter spp. and carbapenem-resistant A. baumannii [7][36]. The varied resistance rates could be explained by prescribing patterns and differences in regional epidemiology. Since 2005, the resistance rate to tigecycline has risen significantly as a result of long-term administration of this drug as a monotherapy and FDA approval.
In 2006, tigecycline was approved by the European Medicines Agency (EMA); in 2011, it was introduced in China. Since 2007, global reports have been published on tigecycline resistance. The Acinetobacter resistant strains had been reported before 2011 [37]. Overprescription of antibiotics has been associated with a higher rate of resistance [38]. There is probably an indirect correlation between the previous use of other antibiotics and tigecycline resistance because of transportation by the similar efflux pump [39]. In addition, treatment by a wide-spectrum antibiotic instead of a narrow-spectrum drug, e.g., due to the inaccurate diagnosis of infection, inappropriate differentiation between virus or bacterium and the resulting improper prescription, as well as self-medication, leads to a growth in drug resistance [40].
The resistance mechanism to tigecycline is mediated by efflux pumps such as AdeABC. The overexpression in AdeABC caused by amino acid and nucleotide changes in the AdeRS two-component system and modified expression of AdeA and AdeB by the BaeSR system is another possible mechanism. In addition, mutations in genes encoding 1-acyl-sn-glycerol-3-phosphate acyltransferase and S-adenosyl-L-methionine (SAM)-dependent methyltransferase result in lower susceptibility [7].
Despite our findings, other review studies indicated conflicting results. In the review of Ni et al. [17], administration of tigecycline was discouraged based on assessment of cohorts and RCT studies. This review demonstrated a higher in-hospital mortality rate, a lower rate of bacterial eradication, and insufficiency of combination therapy in treatment groups compared to the control. Sodeifian et al. applied an approach similar to that used in our work to analyze observational studies. As a result, tigecycline was not recommended for treatment regimens. The researchers found that the overall efficacy of tigecycline in patients was comparable with other antimicrobial agents. Furthermore, a higher death rate and a lower bacterial eradication rate compared to medication regimens based on colistin were established [41].
Care should be taken when interpreting the results due to the limited data. There are few studies on the prevalence of A. baumannii with MDR in Iran, with their majority covering large cities, Tehran in particular. Therefore, more data from other Iranian locations should be obtained to verify the results. In addition, the small size of samples for a subgroup analysis of statistical tests should be considered. There is a high potential of bias when evaluating research studies based on the JBI checklist. None of the studies mentioned confounding factors and the respective corrective approaches; in some studies, statistical methods were not described appropriately. The study setting and validity, as well as the reliability of outcome measurement, remain unclear, thus rendering the interpretation unreliable. Moreover, the clinical samples were obtained from intensive care unit (ICU) patients and individuals hospitalized in various wards, including the burn unit. Variations in isolate extraction methods and the challenging conditions experienced by ICU patients may have potentially impacted the outcomes of antimicrobial susceptibility testing. Additionally, the choice of antimicrobial susceptibility testing methodologies, such as disc diffusion, E-test, or broth microdilution, could also have contributed to discrepancies in results. This variability poses challenges in drawing unequivocal conclusions. This study contributes to the information about the susceptibility of different A. baumannii isolates to tigecycline, thus facilitating a grounded choice of antibiotics for MDR strains. However, further research is needed to obtain more reliable data on the level of such resistance.
CONCLUSION
In the present review and meta-analysis, we evaluated the resistance rate among patients infected by A. baumannii and admitted to hospitals in some cities of Iran. Our findings indicate a high resistance rate of A. baumannii strains against tigecycline; however, tigecycline is still considered an effective drug against MDR bacteria. The meta-analysis results show that the reviewed publications do not provide clear evidence of the overall effect of tigecycline on the resistance rate. In other words, the increase in A. baumannii resistance to tigecycline is not statistically significant, which is confirmed by the results of other studies conducted earlier in Iran.
Increased resistance of A. baumannii to most antibiotics, established in the present study, may be due to improper use or unjustifiably high consumption of broad-spectrum antimicrobial agents, the lack of access to clean water, irregularity of algorithms for sanitary and hygienic and disinfection measures, and administration of antimicrobial combinations in fixed doses, even without knowledge of proven advantages over individual medicinal compounds. There are also social factors, such as self-medication, over-the-counter antimicrobial use, inadequate prevention of infections and diseases, and limited access to high-quality, affordable medicines, vaccines, and diagnostic tools. Given the current situation with the spread of resistant isolates, it is necessary to introduce comprehensive infection control programs aimed at localizing and limiting the spread of A. baumannii strains in medical institutions.
References
1. Jung J, Park W. Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. Appl Microbiol Biotechnol. 2015;99(6):2533-48. https://doi.org/10.1007/s00253-015-6439-y
2. Ahuatzin-Flores OE, Torres E, Chávez-Bravo E. Acinetobacter baumannii, a Multidrug-Resistant Opportunistic Pathogen in New Habitats: A Systematic Review. Microorganisms. 2024;12(4):644. https://doi.org/10.3390/microorganisms12040644
3. Shelburne SA, Singh KV, White AC, et al. Sequential outbreaks of infections by distinct Acinetobacter baumannii strains in a public teaching hospital in Houston, Texas. J Clin Microbiol. 2008;46(1):198-205. https://doi.org/10.1128/jcm.01459-07
4. Vijayakumar S, Rajenderan S, Laishram S, et al. Biofilm Formation and Motility Depend on the Nature of the Acinetobacter baumannii Clinical Isolates. Frontiers in Public Health. 2016;24(4):105. https://doi.org/10.3389/fpubh.2016.00105
5. Sayehmiri F, Alikhani MY, Sayehmiri K, et al. The Prevalence of Antibiotic Resistance to Polymyxins in Clinical Isolates of Acinetobacter baumannii in Iran and the World: A Systematic Review and Meta-Analysis. Shiraz E-Med J. 2017;18(12):e57580. https://doi.org/10.5812/semj.57580
6. Lima WG, Silva Alves GC, Sanches C, et al. Carbapenem-resistant Acinetobacter baumannii in patients with burn injury: A systematic review and meta-analysis. Burns. 2019;45(7):1495-508. https://doi.org/10.1016/j.burns.2019.07.006
7. Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. European Journal of Clinical Microbiology & Infectious Diseases. 2022:1-20. https://doi.org/10.1007/s10096-020-04121-1
8. Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harbor perspectives in medicine. 2016;6(4):a025387. https://doi.org/10.1101/cshperspect.a025387
9. Zhou Y, Chen X, Xu P, et al. Clinical experience with tigecycline in the treatment of hospital-acquired pneumonia caused by multidrug resistant Acinetobacter baumannii. BMC Pharmacology and Toxicology. 2019;20(1):19. https://doi.org/10.1186/s40360-019-0300-3
10. Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022;41(7):1003-22. https://doi.org/10.1007/s10096-020-04121-1
11. Townsend ML, Pound MW, Drew RH. Tigecycline in the treatment of complicated intra-abdominal and complicated skin and skin structure infections. Ther Clin Risk Manag. 2007;3(6):1059-70. PMID: 18516315
12. Navon-Venezia S, Leavitt A, Carmeli Y. High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;59(4):772-4. https://doi.org/10.1093/jac/dkm018
13. Saadati M, Rahbarnia L, Farajnia S, et al. The prevalence of biofilm encoding genes in multidrug-resistant Acinetobacter baumannii isolates. Gene Reports. 2021;23:101094. https://doi.org/10.1016/j.genrep.2021.101094
14. Salehi B, Ghalavand Z, Mohammadzadeh M, et al. Clonal relatedness and resistance characteristics of OXA-24 and-58 producing carbapenem-resistant Acinetobacter baumannii isolates in Tehran, Iran. Journal of applied microbiology. 2019;127(5):1421-9. https://doi.org/10.1111/jam.14409
15. Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrobial Resistance & Infection Control. 2017;6:1-8. https://doi.org/10.1186/s13756-017-0208-x
16. Kaprou GD, Bergšpica I, Alexa EA, et al. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics (Basel). 2021;10(2). https://doi.org/10.3390/antibiotics10020209
17. Ni W, Han Y, Zhao J, et al. Tigecycline treatment experience against multidrug-resistant Acinetobacter baumannii infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;47(2):107-16. https://doi.org/10.1016/j.ijantimicag.2015.11.011
18. Sepahvand S, Darvishi M, Mokhtari M, et al. Evaluation of genetic diversity of colistin-resistant Acinetobacter baumannii by BOX-PCR and ERIC-PCR: the first report. Future Microbiol. 2022;17:917-30. https://doi.org/10.2217/fmb-2021-0225
19. Alavi-Moghaddam M, Dolati M, Javadi A, et al. Molecular detection of oxacillinase genes and typing of clinical isolates of Acinetobacter baumannii in Tehran, Iran. Journal of Acute Disease. 2020;9(1):33-9. https://doi.org/10.4103/2221-6189.274016
20. Salehi B, Ghalavand Z, Mohammadzadeh M, et al. Clonal relatedness and resistance characteristics of OXA-24 and -58 producing carbapenem-resistant Acinetobacter baumannii isolates in Tehran, Iran. J Appl Microbiol. 2019;127(5):1421-9. https://doi.org/10.1111/jam.14409
21. Tafreshi N, Babaeekhou L, Ghane M. Antibiotic resistance pattern of Acinetobacter baumannii from burns patients: increase in prevalence of blaOXA-24-like and blaOXA-58-like genes. Iranian Journal of Microbiology. 2019;11(6):502. https://doi.org/10.18502/ijm.v11i6.2222
22. Yazdansetad S, Najari E, Ghaemi EA, et al. Carbapenemresistant Acinetobacter baumannii isolates carrying blaOXA genes with upstream ISAba1: First report of a novel OXA subclass from Iran. Journal of Global Antimicrobial Resistance. 2019;18:95-9. https://doi.org/10.1016/j.jgar.2018.12.011
23. Salehi B, Goudarzi H, Nikmanesh B, et al. Emergence and characterization of nosocomial multidrug-resistant and extensively drug-resistant Acinetobacter baumannii isolates in Tehran, Iran. Journal of infection and chemotherapy. 2018;24(7):515-23. https://doi.org/10.1016/j.jiac.2018.02.009
24. Zafari M, Feizabadi MM, Jafari S, et al. High prevalence of OXA-type carbapenemases among Acinetobacter baumannii strains in a teaching hospital of Tehran. Acta microbiologica et immunologica Hungarica. 2017;64(4):385-94. https://doi.org/10.1556/030.64.2017.031
25. Sarhaddi N, Soleimanpour S, Farsiani H, et al. Elevated prevalence of multidrug-resistant Acinetobacter baumannii with extensive genetic diversity in the largest burn centre of northeast Iran. Journal of global antimicrobial resistance. 2017;8:60-6. https://doi.org/10.1016/j.jgar.2016.10.009
26. Ansari H, Doosti A, Kargar M, et al. Antimicrobial resistant determination and prokaryotic expression of smpA gene of Acinetobacter baumannii isolated from admitted patients. Jundishapur Journal of Microbiology. 2017;10(11):e59370. https://doi.org/10.5812/jjm.59370
27. Alaei N, Aziemzadeh M, Bahador A. Antimicrobial resistance profiles and genetic elements involved in carbapenem resistance in Acinetobacter baumannii isolates from a referral hospital in Southern Iran. Journal of Global Antimicrobial Resistance. 2016;5:75-9. https://doi.org/10.1016/j.jgar.2015.12.005
28. Pourhajibagher M, Mokhtaran M, Esmaeili D, et al. Antibiotic resistance patterns among Acinetobacter baumannii strains isolated from burned patients. Der Pharmacia Lettre. 2016;8(8):347-51.
29. Jasemi S, Douraghi M, Adibhesami H, et al. Trend of extensively drug-resistant Acinetobacter baumannii and the remaining therapeutic options: a multicenter study in Tehran, Iran over a 3-year period. Letters in applied microbiology. 2016;63(6):466-72. https://doi.org/10.1111/lam.12669
30. Kooti S, Motamedifar M, Sarvari J. Antibiotic resistance profile and distribution of oxacillinase genes among clinical isolates of Acinetobacter baumannii in Shiraz teaching hospitals, 2012- 2013. Jundishapur journal of microbiology. 2015;8(8):e20215. https://doi.org/10.5812/jjm.20215v2
31. Bahador A, Raoofian R, Farshadzadeh Z, et al. The prevalence of ISAba1 and ISAba4 in Acinetobacter baumannii species of different international clone lineages among patients with burning in Tehran, Iran. Jundishapur journal of microbiology. 2015;8(7):e17167. https://doi.org/10.5812/jjm.17167v2
32. Bahador A, Raoofian R, Taheri M, et al. Multidrug resistance among Acinetobacter baumannii isolates from Iran: changes in antimicrobial susceptibility patterns and genotypic profile. Microbial Drug Resistance. 2014;20(6):632-40 https://doi.org/10.1089/mdr.2013.0146
33. Salehi B, Goudarzi H, Nikmanesh B, et al. Emergence and characterization of nosocomial multidrug-resistant and extensively drug-resistant Acinetobacter baumannii isolates in Tehran, Iran. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2018;24(7):515-23 https://doi.org/10.1016/j.jiac.2018.02.009
34. Mulani MS, Kamble EE, Kumkar SN, et al. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front Microbiol. 2019;10:539. https://doi.org/10.3389/fmicb.2019.00539
35. Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292-301. https://doi.org/10.1111/2049-632x.12125
36. Büyük A, Yilmaz FF, Gül Yurtsever S, et al. Antibiotic Resistance Profiles and Genotypes of Acinetobacter baumannii Isolates and In Vitro Interactions of Various Antibiotics in Combination with Tigecycline and Colistin. Turk J Pharm Sci. 2017;14(1):13-8.
37. Korczak L, Majewski P, Iwaniuk D, et al. Molecular mechanisms of tigecycline-resistance among Enterobacterales. Frontiers in Cellular and Infection Microbiology. 2024;14:1289396. https://doi.org/10.3389/fcimb.2024.1289396
38. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic advances in drug safety. 2014;5(6):229-41. https://doi.org/10.1177/2042098614554919
39. Zhong X, Xu H, Chen D, et al. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PloS one. 2014;9(12):e115185. https://doi.org/10.1371/journal.pone.0115185
40. Uddin TM, Chakraborty AJ, Khusro A, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of infection and public health. 2021;14(12):1750-66. https://doi.org/10.1016/j.jiph.2021.10.020
41. Sodeifian F, Zangiabadian M, Arabpour E, et al. Tigecyclinecontaining regimens and multi drug-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Microbial Drug Resistance. 2023;29(8):344-59. https://doi.org/10.1089/mdr.2022.0248
About the Authors
Mohammad RahmanianIslamic Republic of Iran
Mohammad Rahmanian, Student Research Committee,
School of Medicine
Tehran
Mahdiyeh Nozad Varjovi
Islamic Republic of Iran
Mahdiyeh Nozad Varjovi, Student research committee
Tabriz
Niloofar Deravi
Russian Federation
Niloofar Deravi, Student Research Committee, School of
Medicine
Tehran
Zahra Nariman
Islamic Republic of Iran
Zahra Nariman, School of Medicine
Tehran
Amir Gholamzad
Islamic Republic of Iran
Amir Gholamzad, Department of Laboratory Medicine
(Faculty of Paramedical Sciences), Department of
Microbiology and Immunology (Faculty of Medicine), Tehran
Medical Sciences
Tehran
Kimia Keylani
Islamic Republic of Iran
Kimia Keylani, School of Pharmacy
Tehran
Alaleh Alizadeh
Islamic Republic of Iran
Alaleh Alizadeh, Student Research Committee, Faculty of
Medicine, Mashhad Branch
Mashhad
Seyed Reza Mousavianfard
Islamic Republic of Iran
Seyed Reza Mousavianfard, Student Research
Committee, School of Dentistry
Tehran
Supplementary files
Review
For citations:
Rahmanian M., Varjovi M.N., Deravi N., Nariman Z., Gholamzad A., Keylani K., Alizadeh A., Mousavianfard S.R. In vitro evaluation of Acinetobacter baumannii resistance to tigecycline in Iran: a systematic review and meta-analysis. Extreme Medicine. 2025;27(2):257-266. https://doi.org/10.47183/mes.2025-318

















