Scroll to:
The psychophysiological state of a person in altered magnetic conditions
https://doi.org/10.47183/mes.2024-26-3-57-64
Abstract
Introduction. Due to the fact that manned space flights beyond Earth orbit are planned in the near future, it becomes relevant to study the effects of the Earth’s reduced magnetic field on humans.
Objective. To evaluate the features of sensorimotor reactions, quality of night sleep (nocturnal sleep) and the development of daytime sleepiness during a 24-hour stay under hypomagnetic conditions (HMC).
Materials and methods. Experimental studies with the participation of 6 male volunteers aged 26 to 37 years were conducted in 2023. In total, four experimental series were carried out, lasting 24 hours each. The subjects were exposed to hypomagnetic conditions in three groups (the average value was between 0.05–0.14 µT). There was no exposure to such conditions in the placebo group.
The research methods included questionnaires regarding the quality and characteristics of night sleep, daytime wakefulness, as well as the study of sensorimotor functions. Statistical processing was carried out by the Statistica 13.0 software package.
Results. Daytime sleepiness was found to increase under hypomagnetic conditions in 66% of observations as compared to 33% of cases in the placebo series (p=0.003). Immediately following the cessation of experimental exposure, a rapid activation of the central nervous system was observed, which was expressed in a statistically significant decrease in the total visual-motor reaction time.
Conclusions. Under hypomagnetic conditions, the work of brain sleep mechanisms is preserved. Daytime drowsiness that develops under hypomagnetic conditions indicates the possibility of changes in circadian rhythmicity in brain activating systems. The rapid activation of the central nervous system reported immediately following the termination of hypomagnetic conditions has a compensatory character. The revealed features of hypomagnetic conditions influence on the sleep-wake cycle and sensorimotor functions suggest further studies of daytime sleepiness using additional subjective and objective methods of wakefulness level and activity of the central nervous system assessment.
Keywords
For citations:
Kovrov G.V., Popova O.V., Chernikova A.G., Orlov O.I. The psychophysiological state of a person in altered magnetic conditions. Extreme Medicine. 2024;26(3):57-64. https://doi.org/10.47183/mes.2024-26-3-57-64
INTRODUCTION
Hypomagnetic conditions (HMC) of outer space, as well as of the Moon and Mars, represent a potentially critical problem for astronauts’ health and performance during long interplanetary missions. Experiments devoted to the studies of HMC effects on humans have shown a decrease in body reserves due health problems such as sleep disorders, metabolic changes, and the appearance of neurological disorders [1]. The formation of a labor and rest regime in accordance with a person’s physiological capabilities under HMC will allow to increase the adaptation efficiency and fulfillment of the tasks assigned to him/her.
Despite the abundance and diversity of empirical data, the mechanisms underlying magnetoreception are yet to fully identified. As of today, there are many hypotheses — for example, the radical pair model and the magnetoreception model with magnetite (iron oxide) as the key component. Any weakening of the magnetic field represents a stress factor for biological organisms, with the nervous system performing the most important regulatory function in the formation of the organismal stress response [2]. It has been suggested that two types of human nervous system response to the Earth’s magnetic field are the stressor response and sedative effect of slow magnetic oscillations. When studying the reactions of the nervous system when exposed to electromagnetic fields, a nonspecific reaction of brain cells accompanied by inhibition of conditioned reflex activity, including learning and memory processes, was found. It is worth mentioning that the cellular reaction resembled Selye’s stress syndrome [3].
Due to bioethical issues, most experimental studies of HMC effects are carried out on animals. It has been shown that prolonged absence of a magnetic field significantly reduces the ability to adapt [4]. Exposure to HMC significantly impairs neurogenesis and cognitive function in the hippocampus of adult mice by reducing endogenous levels of aminophenyl butyric acid (ABA) in neural stem cells [5]. HMC exposure also produced impairment of noradrenergic activity in the brainstem of golden hamsters, with both the noradrenaline content and the density of noradrenaline-immunopositive neurons significantly decreasing following prolonged exposure to near-zero magnetic environment [6]. Studies of long-term exposure to HMC have shown that animals spend relatively more time learning a new object and its location in space, Zhang B.F. et al. suggest that exposure to HMC impairs spatial and cognitive memory of mice [5]. In addition, leukopenia, low metabolic rate, increased mortality and disruption of circadian rhythms have been found due to the lack of natural magnetic conditions [7]. Changes in circadian rhythm and melatonin secretion are also known to lead to negative consequences in the form of decreased antioxidant capacity of the organism [8].
The HMC effect on humans is less studied. In particular, the ability to solve cognitive tests in humans deteriorates already at 40 min under HMC [9], and the biological effects of HMC depend on the complexity of the task. The maximum effects were observed when performing complex cognitive tests, where the increase in the number of errors ranged from 5.1 ± 1.6% to 7.4 ± 2.5% [10]. The results of the study by Binga V.N. et al. [11] revealed significant changes in cognitive and sensorimotor tests results: a slower reaction speed, increased number of errors and decreased short-term memory of men who were in the “zero magnetic field”. The experiment of Sarimov R.M. et al. [12] in two modes of exposure (“placebo” and “zero magnetic field”) lasting 1 h 17 min with an interval of 40–60 days between series revealed that “zero magnetic field” causes an increase in the number of errors and an increase in the time of task performance in cognitive tests, while the results of cognitive tests under conditions of “zero magnetic field” decreased in 25 out of 40 subjects.
The autonomic regulation of cardiac activity also changed already in the first 8 h of stay in the HMC, mainly due to deviations in the activity of the parasympathetic nervous system [13]. The effects of HMC influence on the development of general asthenia, fatigue, drowsiness, affective reactions and other possible negative psychophysiological states, which pose a threat in terms of emergency situations arising in the course of operator activity, contribute to the disruption of interaction in small groups and complicate their activity, thus reducing the efficiency of space flight target tasks, have not been practically studied.
In 1976, Nakagawa described the occurrence of numerous clinical and subclinical symptoms associated with the weakening of the effect of the Earth’s natural geomagnetic field on humans, which later became known as “magnetic field deficiency syndrome”. In magnetic field deficiency syndrome, loss of work capacity, increased sleepiness, and nighttime sleep disturbances are noted [14]. It can be assumed that HMCs can contribute to the appearance of daytime sleepiness as a condition associated with cognitive deficit and change the quality of night sleep, especially its restorative function, changing the physiological basis for optimal functioning in the sleep-wake cycle.
Quantitative assessment of weak magnetic fields effect on the human body is one of the most debated issues in contemporary magneto- and heliobiology. Since changes in the magnetic field often do not lead to a visible reaction of the organism or significant changes in physiological processes during short-term experiments, the more urgent problem here consists in the assessment of the long-term effect of magnetic field variations.
The aim of the present study was to evaluate the peculiarities of sensorimotor reactions, quality of night sleep, and development of daytime sleepiness during a 24-hour stay in hypomagnetic conditions.
MATERIALS AND METHODS
HMC modeling was carried out in a limited volume by the method of compensation of the Earth’s natural magnetic field by a system of windings with current (Helmholtz rings), whose total magnetic field vector is directed in the opposite direction of the Earth’s geomagnetic field (“Arfa” unit, (IMBP)).
The Arfa facility is designed for modeling of HMC based on the method of compensation of the natural geomagnetic field (GMF), in which the total MF-vector of Helmholtz rings is directed in the opposite direction to the Earth’s magnetic field. A magnetic field uniformly distributed in value and direction is created according to the specially selected diameter of the rings and their location inside the chamber. The system makes it possible to compensate for changes in the magnetic field along the vertical component parallel to the maximum size of the exposure unit.
As a result, the magnetic field induction in the working volume of the movable box of the exposure unit can reach zero and negative values (reverse direction of the GMF-vector). The magnetic field indices are monitored using a three-component FL3-100 sensor (Stefan Mayer Instruments, Germany) [13].
Six healthy male volunteers aged 26 to 37 years (BMI 24.77 ± 2.99) participated in the experiment. All participants underwent medical examination prior to the start of the experiment, were recognized as healthy and had no contraindications for participation in the experiment. All studies were conducted in accordance with the principles of biomedical ethics formulated in the 1964 Helsinki Declaration and its subsequent updates and approved by the Bioethics Commission of the Federal State Budgetary Institution of Science of the State Scientific Center of the Russian Federation — Institute of Medical and Biological Problems of the Russian Academy of Sciences (Moscow) (Protocol No. 641 of 14.06.2023). Each study participant provided a voluntary written informed consent signed by him/her following an explanation of the potential risks and benefits, as well as the nature of the upcoming study. The experiment was a randomized double-blind placebo-controlled study. Each subject participated in 4 experimental series, where one had no exposure to HMC while the other three created HMC with reduced natural magnetic field (mean 0.05–0.14 μT). Thus, 2 experimental groups were distinguished: “Placebo” (n = 6) and “HMC” (n = 18). Each experimental series included 3 sessions (Table 1).
Sensorimotor response studies were performed outside the experimental setup in the first 10 min after the end of session 3.
The study methods included:
- To identify the features of sleep and wakefulness, an original structured questionnaire developed earlier was used; the subjects answered questions related to the sleep-wake cycle. A full description of the questionnaire was previously published [15]. In this study, answers to the following questions were analyzed: occurrence of daytime sleepiness (yes/no), occurrence of daytime sleep (yes/no, how many times per day); bedtime and wake-up time (astronomical time, hour) duration of falling asleep (min), number and duration of night awakenings (min).
- Visual motor integration (VMI) test to assess hand-eye coordination within 10 minutes after the 3rd (afternoon) session with HMC outside the experimental setup. The test was conducted on a hardware-software complex developed at the Institute of Medical and Biological Problems. The test consisted of 11 presentations on the monitor screen of a stimulus in the form of a circle with a diameter of about 2.5 cm. While waiting for the stimulus to appear, the test subject was required to hold the cursor in another area of the monitor screen bounded by a square with a side also about 2.5 cm. When the stimulus appeared, the subject was had to move the cursor as quickly as possible from its location to the area of the circle and click the left mouse button. The results of 11 reactions to the stimulus were used to calculate the mean and standard deviation of the time from the onset of the stimulus to the onset of cursor movement (t1, the sensory component of the response), from the onset of cursor movement to placing the cursor in the circle (t2, the motor component of the response), and from the onset of the stimulus to clicking inside the circle (t3, the total reaction time).
Statistical processing was performed with the help of Statistica 13.0 software package using the nonparametric Mann-Whitney criterion, summary tables (banner tables), sign criterion and analysis of variance. The values of quantitative indicators are given as mean values and errors of mean. The peculiarity of the study was that with a sufficient number of indicators, the number of testers was limited by the peculiarities of its organization (n = 6). Statistical methods acceptable for small samples were used in the study [16].
Table 1. Experimental sessions (8 hours) in a series of studies
|
Experimental sessions and research therein |
|||||
|
1st session (afternoon) in the ARFA unit |
Break, outside 20:00–23:00 |
2nd session (night) in the ARFA unit 23:00–7:00 |
Break, outside 7:00–10:00 |
3rd session (afternoon) 10:00–18:00 |
After the end of the session outside of the ARFA unit 18:00–18:10 |
|
Questionnaire |
Questionnaire (n = 24) |
ПЗМР (n = 24) |
|||
Table prepared by the authors using their own data
STUDY RESULTS
Sleep quality. Analysis of the latent period of sleep (the rate of nocturnal falling asleep) revealed no significant pathological deviations in either experimental group. Prolongation of falling asleep time more than one hour was found in 1 case in 6 nights under placebo conditions and in two cases in 18 nights under HMC exposure. Falling asleep duration from 15 to 30 min under the HMC condition was found in 6 cases over 18 nights; in the placebo condition, this occurred in one case over 6 nights (Table 2).
These results indicate similar responses of sleep duration under both placebo and HMC conditions. At the same time, the relative representation of cases of longer sleep duration (more than 15 min) was slightly higher in the HMC group (Figure 1), although the differences did not reach the level of statistical significance.
During the period of nocturnal sleep under HMC exposure, one case of awakening for 18 nights was noted, while under placebo conditions a similar pattern was registered for 6 nights, which also indicates that there were no differences in the experimental groups in terms of the representation of nocturnal sleep disturbances.
Thus, no pathologic abnormalities were found for the duration of falling asleep and the number of nocturnal awakenings during exposure to HMC.
Daytime sleepiness. When comparing the frequency of daytime sleepiness episodes and/or its absence in the HMC and placebo exposure, an increase in the level of daytime sleepiness under the influence of HMC was revealed in 72% of observations (p = 0.003, according to the criterion of signs) as compared to the placebo group. The corresponding data are presented in Figure 2.
The increase in daytime sleepiness was characterized by the occurrence of daytime sleep in both experimental groups. However, the increase in daytime sleepiness under HMC exposure was not accompanied by an increase in the frequency of daytime sleep episodes as compared to placebo conditions. The data are presented in Table 3.
Single daytime naps were observed in 4 cases in the HMC group and once in the placebo group; double daytime naps were recorded 11 times in the HMC group and 4 times in the placebo condition. In both experimental groups, no daytime drowsiness or daytime sleep was noted in 59% of observations.
Hand-eye coordination after expose of HMC
When evaluating the effect of the end of HMC exposure on рand-eye coordination in the VMI-test, it was found that reaction times t1, t2, and t3 in the placebo group were 342.4 ± 52.9 ms, 455.1 ± 82.5 ms, and 632.5 ± 104.6 ms, respectively, and 336.2 ± 44.1 ms, 433.9 ± 64.1 ms, and 596.0 ± 86.8 ms after the end of HMC exposure.
Examination of VMI-test performance dynamics (Figure 3) revealed that after being in HMC, the values of total task completion time (t3) (except for the 9th presentation) in the HMC exposure group were statistically significantly lower according to the sign criterion M = 590.5 ms (Q25 = 585.7 ms, Q75 = 607.8 ms) vs. M = 644.0 ms (Q25 = 618.8; Q75 = 649.7) after placebo.
The time to move the cursor from the square to the stimulus–motor component of the response (t2) from the 3rd response to the 10th response in the HMC exposure group was lower M = 430.3 ms (Q25 = 425.7 ms, Q75 = 435.5 ms) in comparison with the analogous index of the placebo group M = 458,1 ms (Q25 = 448.2 ms, Q75 = 474.1 ms) and at statistical processing of the data using the criterion of signs the acceleration of reaction time in 9 out of 11 presentations was noted (p < 0.05). The data are presented in Figure 5.
Daytime sleepiness under HMC and sensorimotor functions upon discontinuation
Taking into account the more frequent occurrence of daytime sleepiness in HMC conditions, we compared the reaction time when performing VMI-test in cases where episodes of daytime sleepiness were registered in the past session and where they were not. Analysis was performed using the nonparametric Mann–Whitney criterion. Subjects with identified daytime sleepiness following exposure to HMC had shorter reaction times than subjects exposed to HMC who did not have episodes of sleepiness recorded. In contrast, after placebo sessions, subjects with identified daytime sleepiness had longer VMI-test times than subjects in the placebo group who did not experience sleepiness. The data are presented in Figure 6.
Analysis of variance (F = 22.6, p < 0.05) was performed to assess changes in t1, t2, t3 for the group with reported sleepiness following exposure to HMC and placebo. Figure 7 shows a statistically significant decrease in sensorimotor reaction time immediately after the end of being in the experimental setup in the HMC exposure series compared to placebo.
Analysis of variance (F = 22.6, p < 0.05) was performed to assess changes in t1, t2, t3 for the group with reported sleepiness after exposure to HMC and placebo. Figure 7 shows a statistically significant decrease in sensorimotor reaction time immediately after the end of being in the experimental setup in the HMC exposure series compared to placebo.
Table 2. Duration of falling asleep in experimental groups
|
Conditions |
Falling asleep for less than 15 minutes |
Falling asleep for 15–30 minutes |
Falling asleep for more than an hour |
In total |
|
Number of cases |
||||
|
Placebo |
4 |
1 |
1 |
6 |
|
HMC |
10 |
6 |
2 |
18 |
|
All series |
14 |
7 |
3 |
24 |
Table prepared by the authors using their own data
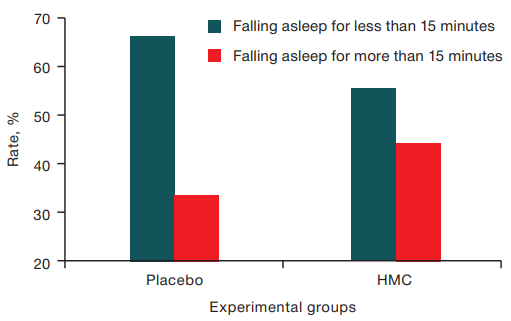
The figure is prepared by the authors using their own data
Fig. 1. Distribution of falling asleep duration values in the experimental groups
Table 3. Episodes of daytime sleep under Placebo and HMC conditions
|
Frequency of sleep disturbances (drowsiness) per day |
Study groups |
|
|
Placebo |
HMC |
|
|
Absent |
7 |
21 |
|
Once |
1 |
4 |
|
Twice |
4 |
11 |
|
Total number of observations |
12 |
36 |
Table prepared by the authors using their own data
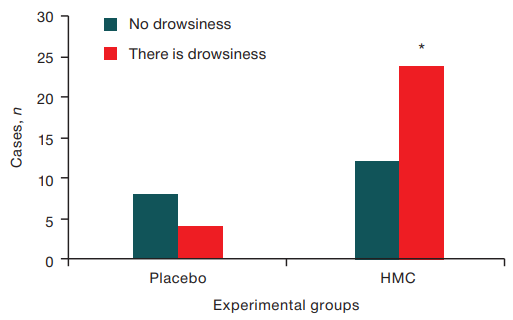
Figure prepared by the authors using their own data
Fig. 2. The presence of daytime sleepiness in Placebo and HMC.
Note: * — p = 0.003
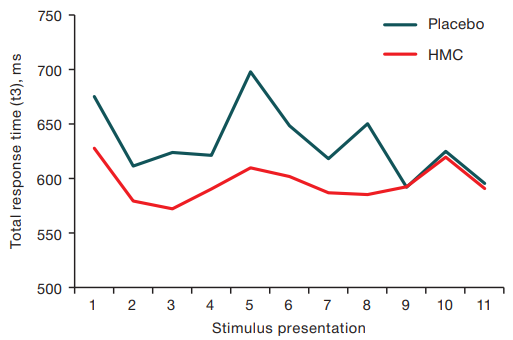
Figure prepared by the authors using their own data
Fig. 3. Time of visual-motor reaction with sequential presentation of stimuli after the end of HMC sessions and Placebo sessions
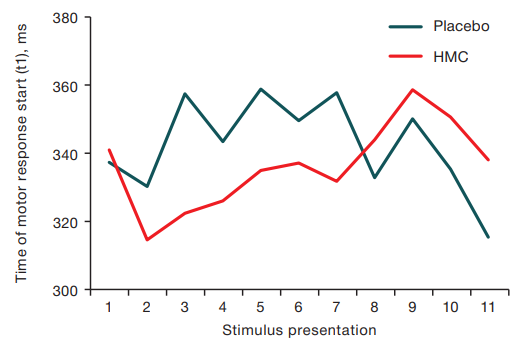
Figure prepared by the authors using their own data
Fig. 4. The time of onset of the motor reaction (t1) with successive presentation of stimuli after the end of the HMC session and Placebo sessions
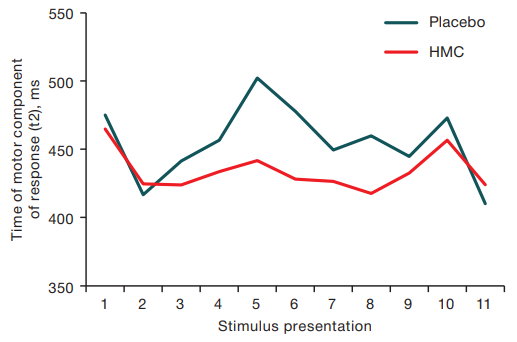
Figure prepared by the authors using their own data
Fig. 5. Cursor movement time to the stimulus (t2) with sequential presentation of stimuli after HMC sessions and placebo sessions
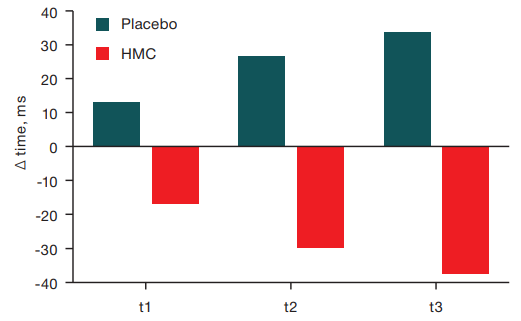
Figure prepared by the authors using their own data
Fig. 6. Sensorimotor functions in groups with and without daytime sleepiness after placebo and HMC conditions
Note: Δ — difference between the values after a session without daytime sleepiness and with daytime sleepiness
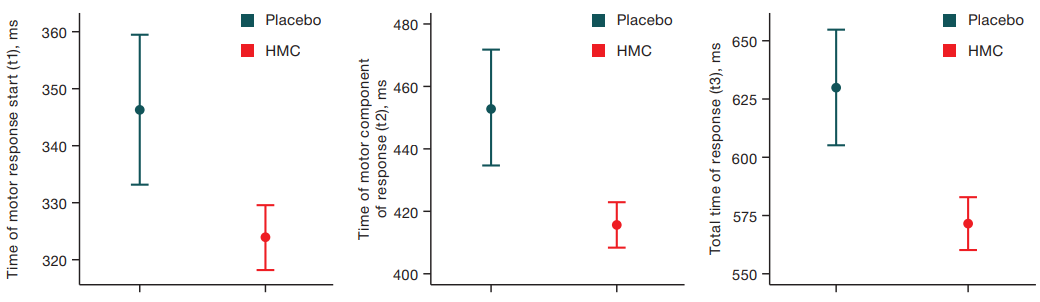
Figure prepared by the authors using their own data
Fig. 7. Results of the variance analysis of VMI-test for the group with daytime sleepiness under placebo and HMC conditions
DISCUSSION
In our study, the detection of daytime sleepiness was based on self-reporting by healthy volunteers who assessed the presence of sleepiness retrospectively after the end of the experimental sessions. This informative approach confirmed the exacerbation of subjective daytime sleepiness under HMC conditions as compared to placebo. The main causes of daytime sleepiness in healthy individuals in most cases are lack of previous nighttime sleep and fatigue state [17]. The appearance of daytime sleepiness and somnolence combined with a reduction in the duration of nocturnal sleep is characteristic of isolation conditions and antiorthostatic tilt [18][19], which also simulate the effect of some spaceflight factors. In studies by Kukanov V. Yu et al. based on changes in the ratios of the sums of delta and theta bands to the sum of alpha and beta bands of the electroencephalogram, a shift in brain activity toward inhibition processes was noted, possibly constituting evidence of the development of fatigue [13]. In turn, we did not detect a reduction in the duration of the preceding night’s sleep, increase in the time taken to fall asleep or an increase in the frequency of night awakenings — i.e., a decrease in its quality, which probably indicates other causes of daytime sleepiness, in particular, the hypnogenic effect of the reduced magnetic field.
The complex symptom of daytime sleepiness, comprised of a feeling of desire to sleep, manifestations of reduced physical and mental activity, ultimately creates additional risks of accident development. It is important to note that sleepiness, which is not a stationary state, can be pronounced or barely noticeable, appear and disappear depending on various external and internal factors, as well as occasionally leading to unexpected falling asleep. The mechanisms of daytime sleepiness development in hypomagnetic conditions are insufficiently studied. Based on the previously revealed drop in the level of norepinephrine in animals under conditions of exposure to HMC [6], we can assume that the neurochemical basis for the development of somnolence in HMC in humans includes a decrease in the level of norepinephrine, acetylcholine, serotonin, dopamine, and other neurotransmitters, whose activity is closely related to the sleep–wake cycle.
The occurrence of daytime sleep and drowsy states may reflect the degree of severity of drowsiness and fatigue and lead to errors in operator activity [17][20]. Our study showed that under the HMC, the subjects had significantly more frequent drowsy states than in the placebo series, while episodes of daytime sleep were observed only during the development of drowsiness, which indicates not only a more frequent but also a more significant disruption of the sleep-wake cycle [21][22] than in subjects with HMC [21][22] than in subjects in the placebo group. Taking into account that daytime falling asleep is an independent medical problem associated with neurochemical changes in orexin production, it can be assumed that the orexin system of wakefulness maintenance is likely to be affected under HMC [23].
In the conducted study the human state was shown to be more active that immediately after the end of a session of HMC exposure than after a placebo session. Already in the first minutes after resuming the action of the geomagnetic field, there is a decrease in the reaction time when performing the VMI-test, which is an interesting phenomenon reflecting the ability of brain systems to rapidly increase neurotransmitter activity [23]. The biological effects of restoring natural geomagnetic field levels are poorly understood. In animal studies [24], it was shown that an 8-day stay of animals in a hypomagnetic environment (30 min per day) resulted in activation of adrenal function one hour after cessation of HMC. The results of our study may reflect these changes, since enhanced adrenal hormone production is directly related to improved function of central nervous system (CNS) and faster reaction times. At the same time, a 30-day stay in the HMC led to a decrease in adrenal function, so the question of the stimulating effect of restoration of the natural magnetic field remains open and requires further study.
It is possible that a person being in a drowsy state plays the role of a kind of “fuse” in the adaptation of the nervous system to the effects of HMC; thus, the restoration of the magnetic field allows the brain to quickly switch to an active state. If this hypothesis is confirmed, then the HMC conditions can be considered as a way of transferring the organism to the level of functioning that is borderline between sleep and wakefulness, where a decrease in cognitive abilities is a protective reaction. In this situation, it is necessary to investigate and develop safe work and rest regimes for individuals in hypomagnetic conditions. Magnetic field reduction can also be considered as an auxiliary means of providing “hibernation” of brain activity, which can be used during flights to deep space. Under terrestrial conditions, hypomagnetic installations can be used in the practice of neuroreanimation when it is necessary to reduce brain activity or in rehabilitation after stressful influences. Restoration of the natural magnetic field, contributing to a rapid increase in brain activity, can be considered as a natural stimulant of cognitive activity.
CONCLUSIONS
- During the nocturnal sleep period, the subjective rate of falling asleep and the frequency of nocturnal awakenings do not change under the influence of HMC, suggesting that brain mechanisms of sleep are preserved in operation.
- Further studies using larger samples, an expanded set of methods, the study of delayed aftereffects of HMC and “fast” effects of restoration of the normal magnetic field are likely to help evaluate the possibilities of using HMC in the therapy and prevention of neurodegenerative and poststress disorders.
References
1. Xue X, Ali YF, Luo W, Liu C, Zhou G, Liu NA. Biological Effects of Space Hypomagnetic Environment on Circadian Rhythm. Front. Physiol. 2021;12:643943. https://doi.org/10.3389/fphys.2021.643943
2. Nikitina EA, Vasilyeva SA, Shchegolev BF, Savvateeva-Popova EV. Weak static magnetic field: Effects on the nervous system. Journal of Higher Nervous Activity. 2022;72(6):783‒99 (In Russ.).
3. https://doi.org/10.31857/S0044467722060077
4. Karpin VA, Kostyukova NK. The influence of weak magnetic fields on higher nervous activity. Baikal Medical Journal. 2004. 46(5):7‒11 (In Russ.).
5. Tian L, Luo Y, Zhan A, Ren J, Qin H, Yongxin Pan. Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus. Int J Mol Sci. 2022; 23(7):3622.
6. https://doi.org/10.3390/ijms23073622
7. Zhang BF, Wang L, Zhan AS, Wang M, Tian LX, Guo WX, Pan YX. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 2021; (12):1174.
8. https://doi.org/10.1038/s41467-021-21468-x
9. Zhang X, Li J F, Wu Q J, Li B, Jiang JC. Effects of hypomagnetic field on noradrenergic activities in the brainstem of golden hamster. Bioelectromagnetics. 2007;(28):155–8. https://doi.org/10.1002/bem.20290
10. Wang X, Jing C, Selby C P, Chiou Y Y, Yang Y, Wu W et al. Comparative properties and functions of type 2 and type 4 pigeon cryptochromes. Cell Mol. Life Sci. 2018;(75):4629–41. https://doi.org/10.1007/s00018-018-2920-y
11. Jia B, Xie L, Zheng Q, Yang PF, Zhang WJ, Ding C, et al. A hypomagnetic field aggravates bone loss induced by hindlimb unloading in rat femurs. PLoS One. 2014;(9):e105604. https://doi.org/10.1371/journal.pone.0105604
12. Sarimov RM, Binhi VN, Milyaev, VA. The influence of geomagnetic field compensation on human cognitive processes. Biophysics. 2008;53(5):433–41. https://doi.org/10.1134/S0006350908050205
13. Binhi VN, Sarimov RM. Zero Magnetic Field Effect Observed in Human Cognitive Processes. Electromagnetic Biology and Medicine. 2009;28(3):310–5. https://doi.org/10.3109/15368370903167246
14. Bingi VN, Milyaev VA, Salimov RM, Zarutsky AA. The influence of electrostatic and "zero" magnetic fields on the psychophysiological state of a person. Biomedical technologies and radio electronics. 2006;8(9): 48‒58 (In Russ.). EDN: HVNYQN
15. Sarimov RM, Binhi VN, Milyaev VA The Influence of Geomagnetic Field Compensation on Human Cognitive Processes. Biofizika. 2008;53(5):856–66. http://doi.org/10.1134/S0006350908050205
16. Kukanov VYu, Vasin AL, Demin AV, Schastlivtseva DV, Bubeev YuA, Suvorov AV et al. Effect of Simulated Hypomagnetic Conditions on Some Physiological Paremeters under 8-Hour Exposure. Experiment Arfa-19. Hum Physiol. 2023;(49):138–46 (In Russ.).
17. https://doi.org/10.31857/S0131164622600343
18. Nakagawa K. Magnetic Field Deficiency Syndrome and Magnetic Treatment. Japan Medical Journal. 1976; 2745
19. Kovrov GV, Vlasova AV, Popova OV, Chernikova AG. Changes in the pattern of sleep disorders in healthy people under conditions of 21-day antiorthostatic hypokinesia ActaBiomedica Scientifica. 2023;8(6):241‒8 (In Russ.).
20. https://doi.org/10.29413/ABS.2023-8.6.24
21. Nosovsky AM, Popova OV, Smirnov YuI. Modern technologies of statistical analysis of medical data and methods of their graphical representation. Aerospace and environmental medicine. 2023;57(5):149‒154 (In Russ.).
22. https://doi.org/10.21687/0233-528X-2023-57-5-149-154
23. Roehrs T., Carskadon M.A., Dement W.C., Roth T. Daytime Sleepiness and Alertness, Principles and Practice of Sleep Medicine. Elsevier. 2017;4:39–48. https://doi.org/10.1016/s0149-7634(87)80016-7
24. Vane AM, Ponomareva IP, Yeligulashvili T, Levin YaI, Kovrov GV, Filimonov MI. Features of the sleep-wake cycle during prolonged isolation. Aerospace and environmental medicine. 1997;31(4):36‒41 (In Russ.).
25. Dorokhov VB. Analysis of the psychophysiological mechanisms of impaired activity during somnolent changes in consciousness. Bulletin of the RGNF. 2003;1(4):137‒44 (In Russ.).
26. Putilov AA, Donskaya OG, Verevkin EG, Arsen'ev GN, Puchkova AN, Dorokhov VB, et al. Overlap between individual variation in personality traits and sleep-wake behavior. Current Psychology. 2021.
27. https://doi.org/10.1007/s12144-021-01495-z
28. Shevtsova KV, Nodel MR, Kachanovsky MS, Kovrov GV, Yakhno NN. Multifactorial daytime sleepiness in Parkinson's disease. Bulletin of the National Society for the Study of Parkinson's Disease and Movement Disorders. 2022;(2):223–6 (In Russ.). https://doi.org/10.24412/2226-079X-2022-12473
29. Nodel MR, Shevtsova KV, Kovrov GV, Yakhno NN. Unexpected falling asleep in patients with Parkinson's disease. Russian Neurological Journal. 2022;27(1):62–8 (In Russ.). https://doi.org/10.30629/2658-7947-2022-27-1-62-68
30. Kovalzon VM. A modern view on the serotonin theory of depression. Russian Neurological Journal. 2020;25(3):40‒4 (In Russ.).
31. https://doi.org/10.30629/2658-7947-2020-25-3-40-44
32. Shust IV, Kostin IM. Reactions of the adrenal cortex of animals to the effects of strong constant MP and hypomagnetic medium. Problems of endocrinology. 1976;22(2):86‒91.
About the Authors
G. V. KovrovRussian Federation
Moscow
O. V. Popova
Russian Federation
Moscow
A. G. Chernikova
Russian Federation
Moscow
O. I. Orlov
Russian Federation
Moscow
Supplementary files
Review
For citations:
Kovrov G.V., Popova O.V., Chernikova A.G., Orlov O.I. The psychophysiological state of a person in altered magnetic conditions. Extreme Medicine. 2024;26(3):57-64. https://doi.org/10.47183/mes.2024-26-3-57-64

















