Scroll to:
Expression of genes SPI1 and GATA3 and Т-helpers subpopulation combination in chronically exposed people
https://doi.org/10.47183/mes.2024-26-3-15-21
Abstract
Introduction. The influence of adverse factors including ionizing radiation leads to a violation of key transcription factors expression and the ratio of the main types of T-helper cells, which in turn initiates a wide range of immunopathological disorders.
Objective. The objective of this research was to study the mRNA expression of the SPI1 and GATA3 genes, as well as the composition of T-helper type 1 and 2 subpopulations, in chronically exposed people during the period of radiation exposure late effects development.
Mаterials and methods. The study was carried out on peripheral blood mononuclear cells obtained from 98 residents of the Techa riverside settlements. Two study groups were formed: the group of exposed individuals (average accumulated dose for the red bone marrow radiation was 706.8±62.7 mGy) and the comparison group (radiation dose did not exceed 70 mGy). The median age of the studied individuals at examination was 71.1 ± 0.9 years (58–87 years). The relative mRNA content of the studied genes was assessed using real-time PCR. The number of T-helpers of types 1 and 2 in the populations of T-helpers of central and effector memory was calculated using the flow cytometry method.
Results. There was a decrease in the absolute and relative number of type 2 T-helpers included in the T-helpers of central memory in chronically exposed individuals. In people with accumulated doses ≥1000 mGy, an increase in the Th1/Th2 ratio of T-helpers of the central memory (p=0.01), as well as the positive correlation relationship between the relative content of type 2 T-helpers of the effector memory and the expression of the GATA3 gene were registered relative to unexposed individuals.
Conclusions. The obtained results indicate that changes in the composition of T-helper cell subpopulations in chronically exposed individuals are not pronounced in the long-term period. However, these changes may directly depend on the total absorbed dose, which in turn determines the prospects for further analysis of the health status of people exposed to chronic high-dose radiation.
Keywords
For citations:
Nikiforov V.S., Kotikova A.I., Akleyev A.V. Expression of genes SPI1 and GATA3 and Т-helpers subpopulation combination in chronically exposed people. Extreme Medicine. 2024;26(3):15-21. https://doi.org/10.47183/mes.2024-26-3-15-21
INTRODUCTION
The human immune system is marked by extremely high radiosensitivity and post-radiation changes in the immune system of exposed individuals can remain for a long time [1][2]. This is most remarkably manifested in persons who have been internally irradiated as a result of deposition of osteotropic radionuclides, in particular Strontium-90 (90Sr), in the body. By accumulating in bone tissue, 90Sr has a prolonged irradiation effect on the red bone marrow (RBM), which is responsible for the formation and development of blood cells, including immunocompetent cells.
Long-term changes in the immune systems of chronically exposed residents of the villages along the banks of the Techa River included a decrease in the expression of differentiation antigens of T-lymphocytes, suppression of T-cells functional activity, a decrease in serum IL-4 level, and an increase in TNFα (tumor necrosis factor-alpha) and IFNγ (interferon-γ). Together, these changes characterize the immune response associated with marked inflammatory reactions [3].
One of the mechanisms involved in the process of immunosuppression found in irradiated individuals is a shift in the balance between cell-mediated immunity (Th1-response) and humoral immunity (Th2-response) [4]. Type 1 (Th1) T-helper cells are responsible for eliminating intracellular pathogens and are associated with organ-specific autoimmune diseases, while type 2 (Th2) T-helper cells initiate immune response to extracellular parasites such as helminths, as well as playing a key role in the development of asthma and other allergic diseases [5]. The balance between T-helper 1 and T-helper 2 plays a key role in the immune response. Normally, cytokines produced by Th2 cells suppress the production of Th1 cytokines and the activity of natural killer cells. In turn, Th1 cells can inhibit the differentiation and proliferation of basophils and eosinophils, whose functions are regulated by Th2 cytokines [6]. However, exposure to high doses of ionizing radiation triggers more pronounced immunosuppressive reactions from the production of Th2-mediated cytokines, while the effect of low doses of radiation on the balance of T-helper cells remains controversial [7][8].
The mechanisms controlling a wide range of T-helper phenotypes, especially under the influence of unfavorable factors, remain poorly understood. Nevertheless, several key genes that play an important role in the regulation of Th1 and Th2 differentiation are currently known [9].
The transcription factor PU.1, encoded by the proto-oncogene SPI1, and the GATA-3 protein, which is encoded by the GATA-3 gene of the same name, are involved in a variety of processes of hematopoiesis and immune system functioning. In immunocompetent cells of the system, these factors are able to trigger the activation of a number of factors such as chemokines, cytokines and cytokine receptors that regulate the processes of differentiation and functioning of T-helper cells. The interaction between PU.1 and GATA-3 controls immune development and homeostasis by regulating the expression of genes specific for Th1 and Th2 subpopulations [10].
Taking into account the above mentioned, the aim of the present work was to study mRNA expression of SPI1 and GATA3 genes, as well as subpopulation composition of T-helpers of types 1 and 2 in chronically irradiated people during the realization of remote consequences of radiation exposure.
MATERIALS AND METHODS
The study was conducted more than 65 years after the beginning of chronic exposure. The object of the study was peripheral blood mononuclear cells obtained from 98 human residents of the villages along the banks of the Techa River. The irradiation of these people was characterized by its low intensity, the value of absorbed doses mainly falling in the range of small- and medium, low intensity and long-term irradiation of red bone marrow (RBM) with pronounced compensatory mechanisms in the hematopoiesis system. Internal irradiation was due to accumulation of radionuclides in the body through consumption of river water and local products. External gamma radiation was caused by contamination of bottom sediments and floodplain soils with radionuclides [11].
The study participants underwent medical examination in the clinical department of Ural Scientific and Practical Center for Radiation Medicine of the Federal Medical and Biological Agency of Russia. Biological material was obtained with the patients’ consent (basis — voluntary informed consent in accordance with the Declaration of Helsinki).
Patients who were in a period of acute or exacerbation of chronic inflammatory diseases, who had oncological and autoimmune diseases, who were taking antibiotics, hormonal and cytostatic drugs during the examination, or who had contact with genotoxic agents in the course of their professional activity, were excluded from the study.
The main dosimetric values determining the measures of ionizing radiation (IR) impact on the organism of the examined persons were personalized radiation doses to the RBM, thymus and peripheral lymphoid organs, as calculated by the specialists of the biophysical laboratory of the Federal state budgetary institute of Ural Scientific and Practical Center for Radiation Medicine of FMBA of Russia using the TRDS-2016 dosimetric system [12].
According to the value of the accumulated radiation dose to the RBM, the patients were divided conditionally into two groups: the comparison group of 45 people, whose dose accumulation occurred during life mainly due to natural radioactive background and medical diagnostic procedures (accumulated doses did not exceed 55 mGy), and the group of 53 chronically irradiated persons, whose average dose to the red bone marrow amounted to 782.0 ± 82.3 mGy.
Patients chronically irradiated were divided into three subgroups depending on the magnitude of the accumulated radiation dose to the RBM.
- Subgroup 1. Irradiated individuals with accumulated doses to the RBM not exceeding 500 mGy (n = 20);
- Subgroup 2. Irradiated persons with accumulated doses to RBM from 500 to 1000 mGy (n = 20);
- Subgroup 3. Irradiated persons with accumulated doses at the RBM of more than 1000 mGy (n = 13).
The ages of the people from all subgroups did not differ statistically significantly. Table 1 presents the characteristics of the studied groups, including the average age of the subjects and the average radiation doses calculated on the RBM, thymus, and peripheral lymphoid organs. The subjects were of Slavic and Turkic origin (Tatars and the Bashkirs).
Blood was collected from the cubital vein in a volume of 3 mL into sterile vacuum tubes Tempus Blood RNA Collection Tubes (Thermo Scientific™, USA) to assess the relative mRNA content of methyltransferases. RNA was isolated by column method using a commercial GeneJET Stabilized and Fresh Whole Blood RNA Kit (Thermo Scientific™, USA). Quantitative and qualitative characteristics of isolated total RNA samples were evaluated using a NanoDrop 2000C spectrophotometer (Thermo Scientific™, USA). The purity of the preparation was assessed by absorbance values at 260 nm and 280 nm wavelengths (A260/280). Reverse transcription reactions were performed as a separate step using a commercial reagent kit MMLV RT kit (Eurogen, Russia). The relative quantitative content of mRNA was determined by real-time PCR using CFX96 Touch amplifier (Bio-Rad Laboratories, USA). Oligonucleotide sequences of primers and probes were developed by the commercial company “DNK-SYNTEZ” LLC. Relative gene production was calculated using the 2–ΔΔCt method, which is based on the calculation of quantitative assessment of expression of genes of interest relative to the “housekeeping gene” based on real-time PCR- analysis [13]. The “housekeeping gene” ACTB (Actin Beta) was used as an endogenous control and data normalization. Calculation was performed using the software of the Real-Time CFX96 Touch instrument (“BioRad”, USA).
A detailed description of the methodology for the study of peripheral blood T-helper subpopulations by flow cytofluorimetry described by Kotikova AI. et al. [14]. The following cell populations were selected for this study: Type 1 T-helpers in the central memory T-helper population (CD3+CD4+CD45RA-CD62L+CXCR5-CXCR3+CCR6-CCR4- phenotype), Type 1 T-helpers in the effector memory T-helper population (CD3+CD4+CD45RA-CD62L-CXCR5-CXCR3+CCR6-CCR4- phenotype), Type 2 T-helpers in the central memory T-helper population (CD3+CD4+CD45RA-CD62L+CXCR5-CXCR3-CCR6-CCR4+ phenotype) and type 2 T-helpers in the effector memory T-helper population (CD3+CD4+CD45RA-CD62L-CXCR5-CXCR3-CCR6-CCR4+ phenotype).
Statistical processing of the results was carried out using SPSS Statistics 17.0 and Graph Pad Prism 8.4.3. The normality of the distribution of quantitative indicators was checked using the Kolmogorov-Smirnov test of agreement. The median (Me) and 25–75th percentiles (Q1–Q3) were used to describe the obtained indicators whose distribution differed from normal. Comparison of data samples was performed using the Mann-Whitney U-criterion due to the distribution of most values not following the law of normal distribution. The Kraskell-Wallis test was used for multivariate comparison. For all criteria and tests, differences were considered statistically significant at p<0.05.
Table 1. Characteristics of the examined persons
|
Parameter |
Comparison Group |
Dose subgroups of exposed persons, RBM dose, mGy |
All exposed persons |
||
|
Subgroup 1 |
Subgroup 2 |
Subgroup 3 |
|||
|
Number of patients, n |
45 |
20 |
20 |
13 |
53 |
|
Age of patients, years, |
68.2 ± 1.0 |
70.5 ± 1.1 |
72.5 ± 0.8 |
72.5 ± 0.6 |
71.7 ± 1.0 |
|
Absorbed dose to RBM, mGy |
17.3 ± 2.3 (0–55.0) |
286.5 ± 27.8 (73.4–485.1) |
695.1 ± 34.8 (509.6–968.6) |
1371.5 ± 71.8 (1032.0–1867.6) |
706.8 ± 62.7 (73.4–1867.6) |
|
Absorbed dose to thymus and peripheral lymphoid organs, mGy |
7.17 ± 1.30 (0–39.52) |
46.4 ± 7.4 (4.6–160.9) |
86.2 ± 16.4 (25.5–354.6) |
165.5 ± 17.7 (103.6–357.9) |
90.6 ± 10.2 (4.6–357.9) |
Table prepared by the authors using their own data
Note: RBM — red bone marrow, N — number of subjects studied; the data are presented as an average value and the standard error of the average (M ± SE) (max-min).
STUDY RESULTS
In the course of the study, statistically significant differences were obtained in the content of type 2 T-helper cells, which are part of central memory T-helper cells in the peripheral blood of exposed subjects and subjects from the comparison group. The corresponding data are presented in Figures 1A and 1B.
Thus, a statistically significant decrease in the absolute number of type 2 T-helper cells was observed in subjects from subgroups with different dose of RBM irradiation compared to the results of subjects from the comparison group, namely: 6.00×109/L (p=0.03) in the total group of exposed subjects, 3.63×109/L (p=0.03) in subjects from the first subgroup, and 5.88×109/L (p=0.02) in subjects from the second subgroup versus 13.14×109/L in subjects from the comparison group. A statistically significant decrease in the relative number of type 2 T-helper cells was also found in the exposed subjects from the subgroups with different dose of BCC irradiation compared to the same index in the subjects from the comparison group (Fig. 1B).
At the same time, a statistically significant increase in the Th1/Th2 ratio in the population of central memory T-helper cells was found in the third subgroup — 14.26%, in irradiated subjects from the general group — 12.54% relative to the index in subjects from the comparison group — 7.25% (Fig. 2). At the same time, in the first and second subgroups of the exposed persons, this index was increased relative to the subjects from the comparison group only at the trend level (p=0.06 and p=0.07, respectively). No statistically significant intergroup differences were found for other studied indicators of the T-helper cells.
Correlation analysis was performed to assess the degree of association between the concentration of T-helper cells, exposure to radiation factors (accumulated radiation doses to RBM, thymus and peripheral lymphoid organs), and the age of patients at the time of examination.
The analysis revealed no statistically significant correlation between the absolute and relative number of type 2 T-helpers in the population of central memory T-helpers and the Th1/Th2 ratio in the population of central memory T-helpers with the RBM cumulative exposure, thymus and peripheral lymphoid organs in the combined study group. There were also no statistically significant correlations between the selected indices of T-helper cells and the ages of the studied individuals whether in the comparison group or in the group of irradiated individuals.
The results of the study of relative expression of SPI1 and GATA-3 genes in the examined patients are presented in Table 2. The data presented in Table 2 indicate that there were no statistically significant differences in the relative mRNA content of SPI1 and GATA3 genes in the individuals from the main group and the comparison group even as the values of the accumulated dose of irradiation of RBM increased.
No statistically significant correlations were found between the relative mRNA content of SPI and GATA3 genes and the RBM radiation absorbed doses, thymus and peripheral lymphoid organs and factors of non-radiation nature (achieved age, sex and ethnicity).
In all chronically irradiated persons, no statistically significant correlations were found between the relative content of SPI1 gene mRNA and the absolute number and frequency of T-helper cells of type 1 and type 2 in the distant terms after the beginning of radiation exposure. At the same time, in the group of irradiated people whose accumulated radiation doses to RBM were in the range of high values (exceeding 1000 mGy), a positive correlation between GATA3 gene expression and the relative content of type 2 effector memory T-helper cells was found (Rs=0.70; p=0.02).
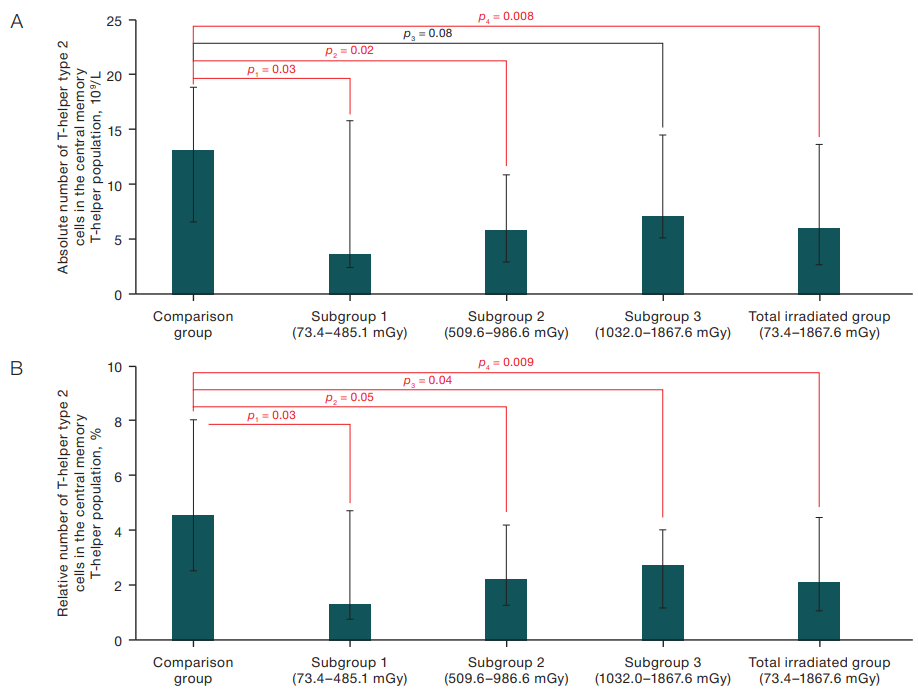
Figures prepared by the author using his own data
Fig. 1. Absolute (A) and relative (B) content of T-helper type 2 cells in the central memory T-helper population in peripheral blood, Me (Q1–Q3)
Note: p — indicates the confidence level of differences relative to the comparison group (Mann-Whitney U test).
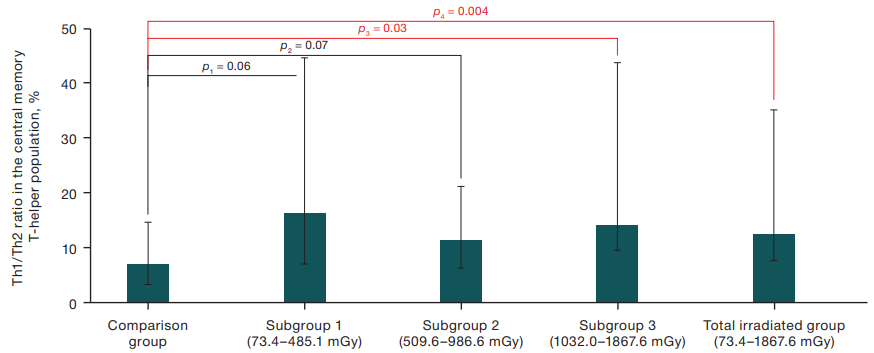
Figure prepared by the author using his own data
Fig. 2. Difference in the Th1/Th2 ratio in the central memory T-helper population between the comparison group and individuals exposed at a wide dose range, Мe (Q1–Q3)
Note: p — indicates the confidence level of differences relative to the comparison group (Mann-Whitney U test)
Table 2. Relative mRNA content of the SPI1 and GATA 3 genes in the studied groups
|
Relative mRNA level of the gene |
Comparison group n = 45) |
Dose subgroups of exposed persons, RBM dose, mGy |
|||
|
Subgroup 1 n = 20 |
Subgroup 2 n = 20 |
Subgroup 3 n = 13 |
All exposed persons (n = 53) |
||
|
SPI1, rel. un. |
1.09 (0.30–2.69) |
1.09 (0.33–3.11) |
1.00 (0.24–2.71) |
0.96 (0.28–2.66) |
1.03 (0.29–2.71) |
|
GATA3, rel. un. |
1.10 (0.05–5.62) |
0.84 (0.01–7.37) |
1.10 (0.19–3.16) |
0.83 (0.02–4.06) |
0.85 (0.02–4.06) |
Table prepared by the author using his own data
Note: The data are presented in Me (Q1–Q3) format
RESULTS AND DISCUSSION
According to earlier data, prolonged exposure to ionizing radiation, even in the range of low doses, can cause a decrease in cellular immunity against the background of changes in the composition of circulating cells of the immune system [15].
T-helper cells representing T-lymphocyte subpopulations play an important role in the regulation of immunity. By mediating the interaction between immunocompetent cells, they determine the type of immune response (cellular or humoral) [16]. In modern literature, there is increasing evidence in favor of radiation-induced changes in the composition and function of T-cell subpopulations, which determines the formation of a specific inflammatory profile of the immune system, disturbance of the balance between Th1 and Th2 subpopulations, and changes in the expression level of transcription factors [17].
The study revealed a statistically significant decrease in the number of type 2 T-helpers in the population of central memory T-helpers in irradiated people. The lowest cellularity indices were observed among individuals whose absorbed doses ranged from 73.4 to 485.1 mGy. In this regard, irradiated individuals showed an increase in the Th1/Th2 ratio in the central memory T-helper population relative to individuals in the comparison group. However, these changes were not related to age or RBM irradiation dose in the thymus and peripheral lymphoid organs. A similar suppression of the Th2 response in mice was demonstrated in an experiment under low-dose radiation exposure conditions [18]. Karimi G. et al., who monitored radiologists with radiation exposure levels of less than 50 mGy, additionally noted a shift in their immune response towards the production of type 1 T-helper cells [19]. Previously, we also described a decrease in the number of type 2 T-helper cells, which are part of central memory T-helper cells, in individuals exposed to chronic low-intensity radiation [20].
Within the framework of the present work, we found no changes in the mRNA expression of the genes of transcription factors SPI and GATA3 in the remote terms after exposure to IR in the residents of the villages along the banks of the Techa River exposed to chronic training. However, it is noteworthy that a statistically significant positive correlation between the relative mRNA content of the GATA3 gene and type 2 effector memory T-helper cells was characteristic of individuals whose absorbed doses of RBM exposure were in the range of high values (more than 1000 mGy). Indeed, GATA3 protein plays an important role in the development of the Th2 phenotype by activating the secretion of cytokines IL-4, IL-5 and IL-13 in Th2 cells, as well as inhibiting specific Th1 transcription factors such as T-bet, NF-AT1, FOXP3 and others [21].
The results obtained in the course of the study, which are consistent with modern scientific data, show that the changes in the immune system in people exposed to chronic low-intensity radiation exposure are not pronounced in the long term. However, further work is required to study the study of key transcription factors involved in the differentiation of immunocompetent cells and their continued functioning in people exposed to high doses.
CONCLUSION
The results of the study recorded a decrease in the absolute number of type 2 T-helper cells, which are part of the central memory T-helper cells, in remote periods of time in individuals exposed to chronic radiation exposure. In individuals with accumulated doses of more than 1000 mGy, an increase in the ratio of Th1/Th2 in the population of central memory T-helpers in relation to unirradiated individuals and a positive correlation between GATA3 gene expression and the relative number of type 2 effector memory T-helpers were observed.
The results obtained confirm the fact that radiation-induced immune changes may directly depend on the total absorbed radiation dose, which determines the prospects for further analysis of the health status of people exposed to chronic high-dose irradiation.
References
1. UNSCEAR Biological mechanisms relevant for the inference of cancer risks from low-dose and low-dose-rate radiation. United Nations Scientific Committee on the Effects of Atomic Radiation. United Nations. New York. 2021.
2. Stewart FA, Akleyev AV, Hauer-Jensen M. Proceedings of the ICRP. Publication 118. ICRP report on tissue reactions, early and late effects in normal tissues and organs — threshold doses for tissue reactions in the context of radiation protection. Chelyabinsk: Kniga;2012 (In Russ.).
3. Akleyev AA. Immune Status of a Man Long after Chronic Radiation Exposure. Medical Radiology and Radiation Safety. 2020;65(4):29–35 (In Russ.). https://doi.org/10.12737/1024-6177-2020-65-4-29-35
4. Chalmin F, Humblin E, Ghiringhelli F, Vegran F. Transcriptional programs underlying Cd4 T cell differentiation and functions. International Review of Cell and Molecular Biology. 2018;(341):1–61. https://doi.org/10.1016/bs.ircmb.2018.07.002
5. Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012;(2012):925135. https://doi.org/10.1155/2012/925135
6. Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/ Th2 lymphocyte polarization in asthma. Allergy. 2000;55(61):6–9. https://doi.org/10.1034/j.13989995.2000.00511.x
7. Han SK, Song JY, Yun YS and Yi SY. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. International Journal of Radiation Biology. 2006;(82):686–97. https://doi.org/10.1080/09553000600930699
8. Gao H, Dong Z, Gong X, Dong J, Zhang Y, Wei W. et al. Effects of various radiation doses on induced T-helper cell differentiation and related cytokine secretion. Journal of Radiation Research. 2018;(59):395–403. https://doi.org/10.1093/jrr/rry011
9. Nikiforov VS., Akleyev AV. mRNA Expression of GATA3, FOXP3, TBX21, STAT3, NFKB1, and MAPK8 Transcription Factors in Humans and Their Cooperative Interactions LongTerm after Exposure to Chronic Radiation. Biology Bulletin. 2022;49(6):588–95. https://doi.org/10.1134/S1062359022060103
10. Rothenberg EV, Hosokawa H, Ungerback J. Mechanisms of action of hematopoietic transcription factor PU.1 in initiation of T-cell development. Frontiers in Immunology. 2019;(10):228. https://doi.org/10.3389/fimmu.2019.00228
11. Akleev AV, ed. Consequences of radioactive contamination of the Techa river. Chelуabinsk: Kniga; 2016. EDN: VYWFDT
12. Degteva MO, Napier BA, Tolstykh EI, Shishkina EA, Bougrov NG, Krestinina LYu et al. Individual dose distribution in cohort of people exposed as a result of radioactive contamination of the Techa river. Medical Radiology and Radiation Safety. 2019;64(3):46–53 (In Russ.). https://doi.org/10.12737/article_5cf2364cb49523.98590475
13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262
14. Kotikova AI., Blinova EA., Akleyev AV. Subpopulation Composition of T-Helpers in the Peripheral Blood of Persons Chronically Exposed to Radiation in the Long Term. Extreme Medicine. 2022; 24(2): 65–73 (In Russ.). https://doi.org/10.47183/mes.2022.018
15. Kodintseva ЕА, Akleyev АА. Delayed correlated parameters of adaptive and innate immunity in chronically irradiated subjects. Russian journal of immunology. 2020;23(2):225–30 (In Russ.). https://doi.org/10.46235/1028-7221-399-DCP
16. Alberts B, Johnson A, Lewis J et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Helper T Cells and Lymphocyte Activation. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26827.
17. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mammalian Genome. 2018;29(11):843–65. https://doi.org/10.1007/s00335-018-9777-0
18. Gao H, Dong Z, Gong X, Dong J, Zhang Y, Wei W,et al. Effects of various radiation doses on induced T-helper cell differentiation and related cytokine secretion. Journal of radiation research. 2018;59(4):395–403. https://doi.org/10.1093/jrr/rry011
19. Karimi G, Balali-Mood M, Alamdaran SA, Badie-Bostan H, Mohammadi E, Ghorani-Azam A, Sadeghi M, & Riahi-Zanjani B. Increase in the Th1-Cell-Based Immune Response in Healthy Workers Exposed to Low-Dose Radiation — Immune System Status of Radiology Staff. Journal of pharmacopuncture. 2017;20(2):107–11. https://doi.org/10.3831/KPI.2017.20.014
20. Nikiforov VS, Kotikova AI, Blinova EA, Akleyev AV. Transcriptional Activity of Genes Regulating T-Helper Differentiation in the Accidentally Exposed Population of the Southern Urals. Dokl Biochem Biophys. 2024. https://doi.org/10.1134/S1607672924701114
21. Shih H-Y, Sciume` G, Poholek AC, Vahedi G, Hirahara K, Villarino AV et al. Transcriptional and epigenetic networks that drive helper T cell identities. Immunological Reviews. 2014;261(1):23–49. https://doi.org/10.1111/imr.12208
About the Authors
V. S. NikiforovRussian Federation
Chelyabinsk
A. I. Kotikova
Russian Federation
Chelyabinsk
A. V. Akleyev
Russian Federation
Chelyabinsk
Supplementary files
Review
For citations:
Nikiforov V.S., Kotikova A.I., Akleyev A.V. Expression of genes SPI1 and GATA3 and Т-helpers subpopulation combination in chronically exposed people. Extreme Medicine. 2024;26(3):15-21. https://doi.org/10.47183/mes.2024-26-3-15-21
















