Scroll to:
Study of complications associated with central vein catheterization in patients with blood disorder
https://doi.org/10.47183/mes.2024-26-3-106-112
Abstract
Introduction. Central venous catheter (CVC) provides intensive infusion and transfusion therapy in cancer patients, but catheter placement and operation are often associated with complications.
Objective. To determine the incidence of complications associated with CVC in patients with blood disorders.
Materials and methods. The study involved 3115 patients and 46 bone marrow donors. The right subclavian vein was catheterized in 2600 (82.2%) patients, the left subclavian vein in 552 patients (17.5%), and the internal jugular vein in 9 patients (0.3%). All persons underwent radiologic control; bacteriological blood examination was performed in case of suspected infection.
Results. Early revealed complications were: hematoma in 4.0% of patients; bleeding — in 2.3%; subclavian artery puncture — in 2.7%; pain and paresthesia of the upper limb — in 1.7%; lymphorrhea — in 1.4%; weakness / collapse — in 1.2%; extravasation — in 1.1%; catheter thrombosis — in 1.1%; less frequently, pneumothorax was detected in 0.2% of patients; allergic reaction to anesthetic — in 0.1%. Delayed complications (infiltrate, phlebitis, thrombophlebitis) were diagnosed in 2.7% of patients, bacteremia — in 2.4%, delayed bleeding — 0.4%. Among infections, Gram positive microorganisms were more frequently detected in 61.8% of cases, Gram negative in 29.7% (p < 0.01), and rarely fungal pathogens in 8.5% (p < 0.001). It was not possible to catheterize the central vein due to anatomical features of the patient in 0.5% of cases.
Conclusions. The analysis of trunk vein catheterization in patients with blood disorders established a high rate of hematomas, bleeding, subclavian artery punctures; among delayed complications, infiltrate, phlebitis, and bacteremia. Infectious complications demonstrated a prevalence of Gram-positive infectious agents.
Keywords
For citations:
Romanenko N.A. Study of complications associated with central vein catheterization in patients with blood disorder. Extreme Medicine. 2024;26(3):106-112. https://doi.org/10.47183/mes.2024-26-3-106-112
INTRODUCTION
Managing patients with blood disorders at present often requires adequate venous access, which can be provided through the use of central venous catheters (CVCs). The use of CVCs in patients allows the introduction of various infusion media, hemocomponents, chemotherapy drugs directly into the lumen of the central vein. This provides the possibility of administering large volumes of fluid and round-the-clock infusion of drugs, parenteral nutrition, hematopoietic stem cell harvesting, as well as monitoring of central venous pressure and clinical and laboratory parameters. At the same time, administration of chemotherapy drugs into the peripheral vein does not exclude their extravasation into the surrounding soft tissues, with the risk of infiltrate or necrosis development and subsequent scar formation [1].
Provision of central venous access using CVCs in patients in intensive care units has been widespread since the 1970s. This procedure makes possible a quick refilling of circulating blood and plasma volume in case of blood loss and shock, significantly improving the quality of life of patients due to the absence of the need for multiple repeated venipunctures, particularly in patients with low venous pressure and weak peripheral veins [1–2].
The procedure of CVC placement, which refers to surgical interventions, is often associated with the following complications: hematoma, bleeding, arterial puncture, pneumothorax, venous thrombosis, secondary infections up to the bacteremia or sepsis; in some port-systems, placement or even detachment of the implanted catheter with its migration into the right atrium can occur [2–6]. According to some authors [2][3][7], the incidence of complications associated with central vein catheterization reaches 15–25%. This depends on the anatomical and topographical features of the vessel structure, the state of the hemostasis system in the patient, as well as the proper technique of CVC placement. In addition, the risk of thrombotic and infectious complications increases with prolonged use of venous catheter especially during the period of antitumor therapy. Therefore, in patients with hemostasis disorders (thrombocytopenia, disseminated intravascular coagulation — DIC), immunodeficiency, neutropenia, compliance with aseptic conditions at all stages of CVC operation, including the use of aseptic technique ANTT (Aseptic Non-Touch Technique) is particularly strictly required [8–10].
This study aims to investigate the incidence of central venous catheter-associated complications in patients with blood disorders.
МАТЕRIALS AND METHODS
A retrospective study was conducted to investigate the incidence of complications associated with CVC placement at RosNIIGT of FMBA in Russia. The study included patients aged 18 years and older with blood disorder who signed an informed voluntary consent for CVC placement. Healthy donors of hematopoietic stem cells were additionally included in the study. Patients were excluded who refused to sign informed consent, persons under the age of 18 years, and those who were not diagnosed with oncohematological diseases or aplastic anemia.
Inpatient records of 3161 patients who underwent central vein catheterization and were treated at the hematology clinic of the institute for the period from 2003 to 2023 were studied. Of these, 3115 patients with blood diseases and 46 bone marrow donors were examined.
The age of the patients was 18–89 years (mean age = 53 years) and donors were 29–57 years (mean age = 41 years). Patients who underwent CVC staging had principal diagnoses of B-cell chronic lymphocytic leukemia (B-CLL), myelodysplastic syndrome (MDS), multiple myeloma (MM), primary myelofibrosis (PMF), and chronic myeloid leukemia (CML) in the blast crisis phase (CML-BC), myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN), non-Hodgkin’s lymphoma (NHL), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), Hodgkin’s lymphoma (HL), and aplastic anemia (AA). The distribution of patients with CVC by relevant nosologies is presented in Figure 1.
As anesthesia, 1% lidocaine solution of 10–15 mL (in 99.1% of patients) or 0.5% novocaine solution of up to 10–15 mL (0.9% of patients) was used. CVC placement was performed in the right subclavian vein in 2,600 (82.2%) patients, in the left subclavian vein in 552 (17.5%), and in only 9 (0.3%) patients in the right internal jugular vein. The left subclavian vein or right internal jugular vein was catheterized in patients with a history of previous failed attempts at CVC placement or technical difficulties in performing the manipulation at the moment.
The choice of central venous catheter was made taking the choice of further therapies into account: chemotherapy, hemotransfusion, harvesting of peripheral stem cells, or the volume of expected infusions, duration of treatment, etc. B.BRAUN (Germany) Certofix® Duo S 720 for patients with blood disorders and Certofix® Duo RA M 1220 for stem cell donors were used for catheterization of the main veins.
The effectiveness of the central vein catheterization procedure was assessed by retrograde blood flow into the syringe, visual inspection, radiography; if necessary, the procedure was performed under ultrasound data control. The figures show radiographs to visualize the terminal (distal) segment of the catheter location. The normal location of the catheter is shown below (Fig. 2).
The vertical position of the CVC of the end segment in the internal jugular vein was confirmed radiologically (Fig. 4). If the CVC was upright, it was often retained for short-term therapy. However, if peripheral stem cell procurement was required or high-dose chemotherapy was planned, the CVC was removed and placed on the opposite side due to the risk of phlebitis and thrombosis.
In case of fever (>38°C) or suspicion of infections associated with CVC, blood was effused directly from the patient’s vein or catheter with subsequent bacteriological analysis, for which blood was introduced into one or more vials with nutrient medium (for blood — thioglycolate medium; for a biosample from the catheter — sugar broth, Sabouraud’s medium, or thioglycolate medium). To identify the pathogen and determine the sensitivity of microorganisms to antibacterial medicines, the biomaterial was transferred to differentiating media. In addition, the level of procalcitonin in blood was studied in order to predict the possible development of bacterial sepsis in the shortest possible time and differentiate the infectious process, depending on the composition of anaerobic and aerobic microflora. If infection associated with CVC was detected, the catheter was removed and antibacterial therapy was performed.
All complications associated with CVC are divided into those directly related to the procedure of catheter placement and delayed complications, i.e., related to catheter care and disorders in the system of hemostasis, immunity.
Statistical processing of the obtained data was performed using Microsoft Office Excel 2010 and SPSS Statistic 22 software packages in the Windows 2016 environment. Samples were tested for normality of distribution using the Kolmogorov-Smirnov test of agreement; the level of statistical significance p > 0.2 was considered reliable. The mean (M), standard deviation (m), median (Me) were calculated. Fractions were compared using Fisher’s angular transformation (φ). Differences were considered statistically significant at p < 0.05.
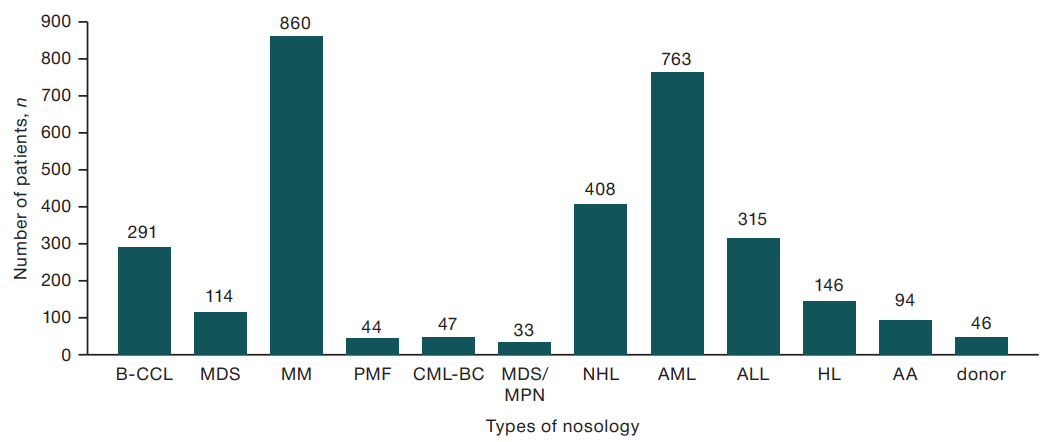
Figure prepared by the author using his own data
Fig. 1. Distribution of patients with established CVC depending on the disease
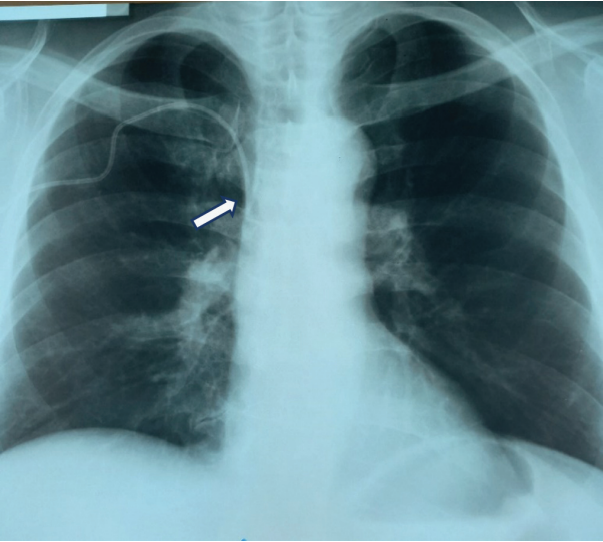
Figure prepared by the author using his own data
Fig. 2. Chest X-ray of the patient after correct catheterization of the right subclavian vein (arrow shows the catheter’s distal segment)
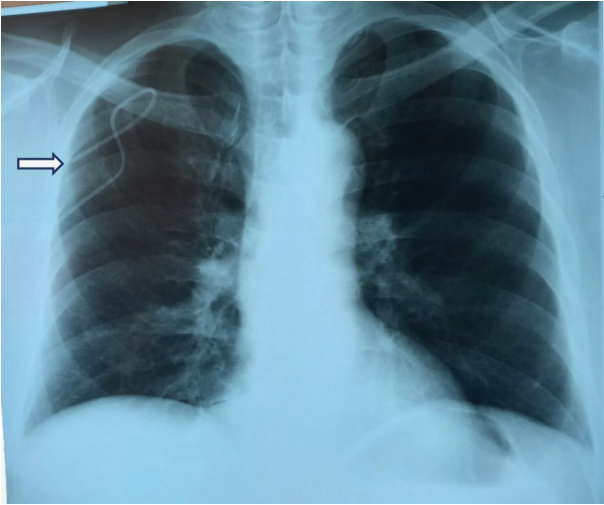
Figure prepared by the author using his own data
Fig. 3. X-ray of chest: extravasation of the central venous catheter — outside the subclavian vein (the catheter is localized outside the subclavian vein)
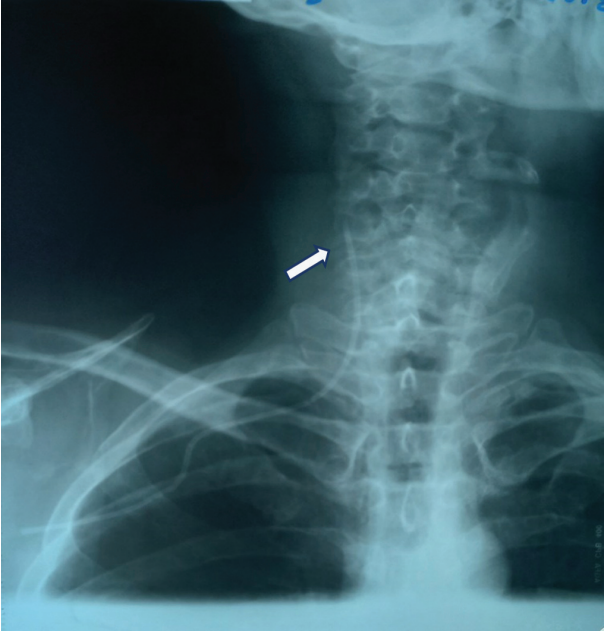
Figure prepared by the author using his own data
Fig. 4. X-ray of the neck and upper chest of the patient after CVC placement from the right subclavian access — vertical placement of the catheter in the internal jugular vein (the arrow shows the distal segment of the catheter)
RESULTS
In 46 healthy bone marrow donors, no clinically significant complications related to central vein catheterization were documented in any case. Only one patient had weakness on anesthetic injection (lidocaine); this symptom was resolved independently 15 min after catheterization. In 46 healthy bone marrow donors, the duration of CVC in the vein was 1.2±0.1 days (1–3 days).
In patients with blood diseases (n = 3115), complications directly related to the technique of placement were observed, mainly mechanical or hemostasis disorders (bleeding, hematomas, thrombosis), delayed complications associated with the accession of secondary infection due to defects in the care of CVCs and/or immune system in patients. Among the complications reported at the stage of CVC insertion or after its implementation were arterial punctures, hematomas, bleeding from the catheter passage, pneumothorax, pneumomediastinum, hemoptysis, catheter malposition (extravasation), lymphorrhea, pain, paresthesia on the side of catheterization, vascular (general weakness, collapse), and allergic reaction (urticaria, Quincke’s edema) to the anesthetic, as well as infectious-inflammatory — infiltrate, phlebitis, thrombophlebitis, bacteremia, and sepsis. Catheter bending (knot formation), disruption of catheter integrity, and catheter obstruction (thrombosis) are also listed as adverse events. While the latter do not constitute complications for the patient, further infusion through such a catheter is impossible and removal of the CVC is required. In addition, 16 (0.5%) patients failed to place the catheter due to anatomical peculiarities of vessel location in the patient.
The nature and frequency of complications associated with CVC placement depended on the anatomical features of the patient’s vascular structure, technique of CVC placement, vascular access, physician’s experience, peculiarities of the course of the disease itself (cytopenia, coagulopathy), and catheter care. A total of 702 adverse events were reported, representing 22.5%. Data on complications associated with CVC placement and care are presented in Table 1.
An analysis of the data in Table 1 revealed a low incidence of complications in patients. Attention was drawn to the frequent development of hematomas in 4.0% of cases (p < 0.01), bleeding from the catheter site, immediate and delayed bleeding 2.3% (p < 0.05) and 0.4%, respectively, registered subclavian artery puncture in 2.7% of cases (p < 0.01), development of infiltrate, phlebitis, thrombophlebitis in 2.7% of cases (p < 0.01), and bacteremia or sepsis in 2.4% of patients (p < 0.01). Other complications were reported significantly less frequently. Such dangerous complications as pneumothorax and pneumomediastinum were detected only in 6 (0.2%) and 1 (0.03%) patients. When diagnosing tension pneumothorax, it is necessary to drain the pleural cavity to evacuate air therefrom.
A radiograph of a 52-year-old patient with bilateral tension pneumothorax is presented. This is a rare but potentially dangerous complication: there is no lung pattern in the upper parts of both sides of the chest (Fig. 5). The patient underwent pleural cavity drainage in the 6th intercostal space along the mid axillary line on both sides for decompression. Full recovery occurred on the 2nd day. The incidence of such complications during CVC placement is less than 1%.
The manifestation of complications in the form of infectious processes was noted by the presence of infiltration with hyperemia at the catheter entry site, sometimes with exudate separated from the wound, soreness at the site of CVC insertion, as well as the appearance of thickening or edema at the site of CVC entry and/or along its length with increasing soreness. In case of deep spread of the infectious process, phlebitis was registered, manifested by erythema, thickening, pain along the course of the vein where the CVC was located, and increased body temperature in the patient. In some patients, phlebitis turned into bloodstream infection with the development of bacteremia or sepsis.
Thus, the analysis revealed the development of bloodstream infections in patients 3–7 days after catheterization. These infections were most frequently caused by Gram-positive microflora: either coagulase-negative Staphylococcus epidermidis, probably due to a defect in the care of CVCs, or, in later periods coagulase-positive Staphylococcus aureus. Infections due to Gram-negative pathogens and fungi (Escherichia coli, Enterobacter spp. etc.) were probably caused by penetration into the body during catheter use during infusions, as well as by hematogenous pathway (not related to CVC placement). In 76 patients, it was possible to identify the pathogen by bacteriological examination of blood; the corresponding data are presented in Table 2. At the same time, bacteriological examination in 13 patients allowed the simultaneous isolation of 2–3 pathogens, which in total amounted to 94 cases of positive identification of a microbial culture.
Among the infectious pathogens isolated from hemoculture in patients with complications, Gram-positive microorganisms (Staph. Epidermidis and Staph. Aureus, etc.), significantly less frequently in 29.7% of cases (p < 0.01) — Gram-negative (Escherichia coli, Enterobacter spp., etc.), in 8.5% of cases (p < 0.001) infection was caused by fungal pathogens (Candida albicans, etc.). For clarity, the main spectrum of detected microorganisms is presented in Fig. 6.
Thus, in the course of the study, hematomas, bleeding, and subclavian artery puncture were more frequently reported among the immediate complications after CVC placement, while among the delayed ones (after 3–7 days) — infiltrate, phlebitis, thrombophlebitis, and bloodstream infections.
Table 1. Complications associated with CVC placement and care (n = 3115)
|
Type of complications |
Number of complications (n = 702) |
Frequency of complications (%) |
|
Puncture (puncture) of the subclavian artery |
85 |
2.7♦ |
|
Hematoma |
124 |
4.0♦ |
|
Bleeding that occurred immediately after CVC placement |
71 |
2.3* |
|
Delayed bleeding (2 hours more) |
12 |
0.4 |
|
Pneumothorax |
6 |
0.2 |
|
Pneumomediastinum |
1 |
0.03 |
|
Hemoptysis |
2 |
0.06 |
|
CVC extravasation |
35 |
1.1 |
|
Lymphorrhea |
43 |
1.4 |
|
Pain, paresthesia in the upper extremity |
53 |
1.7 |
|
Weakness, collapse (reaction to anesthetic) |
38 |
1.2 |
|
Allergic reaction to anesthetic |
3 |
0.1 |
|
Infiltrate, phlebitis, thrombophlebitis |
83 |
2.7♦ |
|
Bacteremia, sepsis |
76 |
2.4♦ |
|
Bend of catheter |
6 |
0.2 |
|
Defect of catheter integrity (crack, fracture of CVCs) |
14 |
0.4 |
|
Catheter obstruction (thrombosis) |
34 |
1.1 |
|
Impossibility of CVC placement (anatomical peculiarities) |
16 |
0.5 |
Table prepared by the author using his own data
Note: ♦ — statistically significant difference from background values at p < 0.01
* — statistically significant difference from background values at p < 0.01
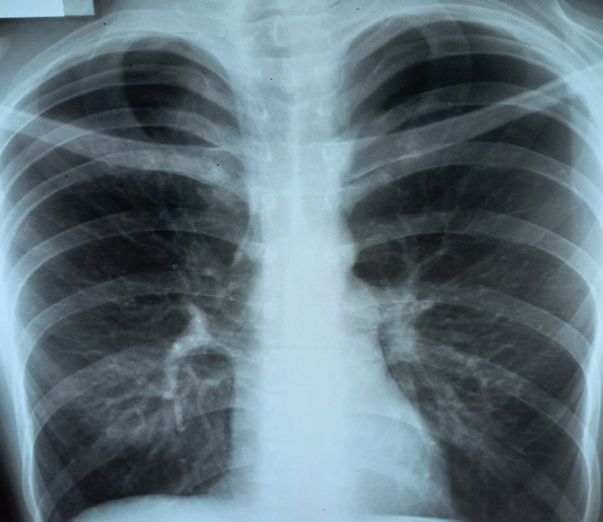
Figure prepared by the author using his own data
Fig. 5. X-ray of patient’s chest — bilateral tension pneumothorax
Table 2. Types of pathogens identified in patients with blood diseases (n = 76)
|
Pathogens |
Number of positive tests, n |
Ratio of positive tests isolated, % |
|
|
Gram-positive microbes |
Staphylococcus epidermidis |
47 |
50.0 |
|
Staphylococcus aureus |
8 |
8.5 |
|
|
Streptococcus viridance |
1 |
1.1 |
|
|
Enterococcus spp. |
1 |
1.1 |
|
|
Micrococcus spp. |
1 |
1.1 |
|
|
Gram-negative microbes |
Escherichia coli |
14 |
14.9 |
|
Enterobacter spp. |
9 |
9.5 |
|
|
Acinetobacter spp. |
1 |
1.1 |
|
|
Pseudomonas aeruginosa |
2 |
2.1 |
|
|
Neisseria spp. |
2 |
2.1 |
|
|
Fungal infections |
Candida. albicans |
5 |
5.3 |
|
Candida crusei |
1 |
1.1 |
|
|
Rhodotorula spp. |
1 |
1.1 |
|
|
Aspergillus spp. |
1 |
1.1 |
|
Table prepared by the author using his own data
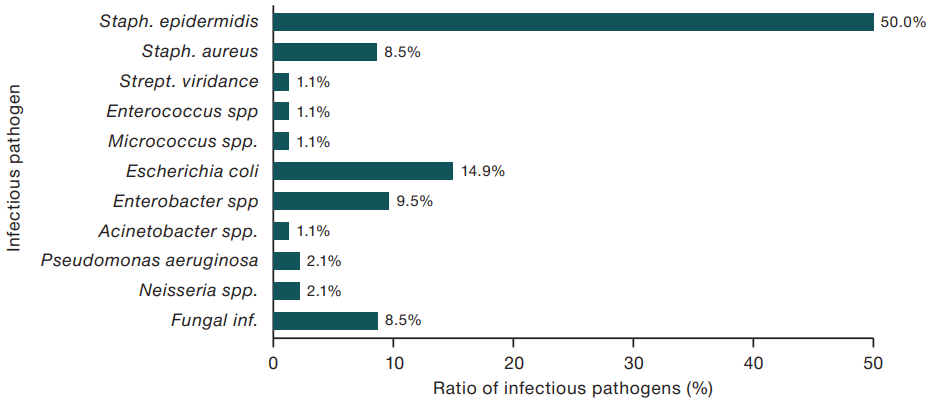
Figure prepared by the author using his own data
Fig. 6. Pathogens associated with central venous catheter-associated infections
DISCUSSION
The availability of central venous access for the administration of adequate therapy in oncohematological patients is of great importance. This allows the physician to perform daily infusion therapy, administer large volumes of infusion media, high-dose chemotherapy, and parenteral nutrition. However, despite the importance of central vascular access for facilitating the administration of drugs, there is a risk of complications that can occur during the catheterization procedure especially with a prolonged stay of the catheter in the vein. The incidence of particular complications occurring during CVC placement is highly variable, ranging from 2.7% to 8% (mean 4.5%); however, aggregate incidence can even reach up to 15–25% [2][3][7-9]. The incidence of complications is influenced by factors such as the type of CVC (port or with an external segment), puncture access (occurs less frequently in subclavian or jugular vein catheterization), prior catheter placement, pathophysiological conditions (immunodeficiency, cytopenia, coagulopathy) and anatomical and topographical features of the structure in the patient, experience of the anesthesiologist or surgeon, and conscientiousness in identifying and documenting the occurrence of the complication [2][8][9]. For example, when a port system is used, the cumulative incidence of early complications reaches 3.28% [11]. In addition, there are cases of failure of central vein catheterization; moreover, their frequency increases with each repeated unsuccessful attempt: after the first attempt — by 1.6%; after the second — by 10.2%, after the third and more — by 43.2% [12]. During the study, no catheterization of any subclavian vein could be carried out in 16 (0.5%) of the total number of patients; for these patients, infusion therapy was administered through peripheral veins.
When analyzing the frequency of complications associated directly with the procedure of catheter placement, the occurrence of hematomas was statistically significantly more frequent in 4% of patients, while bleeding from the site of CVC catheterization was slightly less frequent in 2.7% (immediately after the procedure — 2.3%; delayed — 0.4%; 2 hours or more after catheterization, subclavian artery puncture occurred in 2.7% of patients). It is important to emphasize that the occurrence of hematomas and bleeding in patients with blood diseases is more frequently caused by hemostasis disorders: such catheterized patients more often suffer from severe thrombocytopenia (mainly IV or III severity), and less often — from coagulopathy. Arterial puncture as a complication was mainly due to anatomical features in patients “keel-shaped chest”, pronounced edema syndrome, obesity, clavicle fractures (in the past), presence of tumors/enlarged lymph nodes in the subclavian and/or supraclavicular region or in the neck region. These complications have generally been resolved without additional surgical interventions. In cases of severe thrombocytopenia, hemostatic therapy and transfusions with platelet concentrate at a therapeutic dose of 200–250×109 platelets per 1 m2 of body surface area (usually 200–300 mL of platelet concentrate per injection) were carried out. In most cases, collapse, lymphorrhea, pain, and paresthesia did not require additional medical interventions and were managed independently. However, if the patient was found to have massive lymphorrhea lasting more than a day, the CVC was removed, followed by a sterile dressing. In case of patency failure (thrombosis), integrity, bending or extravasation, the catheter was removed and, if necessary, reinserted more often from the opposite side or via jugular access.
According to some authors, the frequency of detection of such a formidable complication as pneumothorax can reach 0.3–1.0% of cases [3][13]. In our study, pneumothorax and pneumomediastinum were diagnosed in 0.2% and 0.06% of cases, respectively, which required additional medical interventions, in particular, drainage of the pleural cavity. Complications associated with CVC placement as manifested by the development of inflammation depend not only on compliance with the rules of asepsis, but also on the immune status of the patient (presence of severe neutropenia), the type of venous catheter. Thus, according to D.G. Maki and co-authors, when using central venous catheters with an open segment, infectious-inflammatory complications amounted to 2.7 per 1000 catheter-days, while the use of port-systems — no more than 0.1 per 1000 catheter-days [14]. In the patients observed in the present study, complications associated with infectious-inflammatory reaction in the form of infiltrate, phlebitis or thrombophlebitis were diagnosed in 2.7% of cases, while bloodstream infection occurred in 2.4% of cases, which amounted to 3.1 per 1000 per catheter-days. This is higher than in non-oncologic patients. However, the studied cohort of patients with oncohematological diseases and aplastic anemia often have a defect of hematopoiesis as manifested by postcytostatic immunosuppression and neutropenia of III-IV severity and a consequently higher risk of infectious complications. Therefore, if an infectious process is suspected, microbiological examination should be performed and empirical antibacterial therapy prescribed. In the case of positive bacteriologic analysis or identification, the catheter should be removed due to the possibility of comprising a source of infection; if necessary, the antibacterial drug sensitive to the identified pathogen should also be changed [1].
CONCLUSIONS
- The analysis of catheterizations of the main veins established a higher frequency of such early complications as hematomas, bleeding caused by hemostasis disorders (thrombocytopenia, coagulopathy) in oncohematological patients, as well as punctures of the subclavian artery due to anatomical features of the main vessels due to compression and displacement by tumor mass of lymph nodes and changes in the walls of subclavian veins associated with frequent repeated catheterizations and resulting scars.
- Infiltrates, phlebitis, bacteremia, or sepsis were more often stated among delayed complications. Among the infectious pathogens isolated during bacteriological examination of hemoculture, Gram-positive pathogens prevailed significantly, which could be due not only to neutropenia in the study subjects, but also to contamination of infections from the environment during the operation and care of the venous catheter.
- Given the high risk of complications associated with CVCs in the category of oncohematological patients with neutropenia, immunodeficiency requires strict compliance with aseptic conditions and measures to prevent catheter-associated infections at all stages of its operation.
References
1. Abdulkadyrov KM, Shmidt AV. Central venous catheters in hematology: priorities and problems. In the book Hematology: The Newest Handbook. M.: Publishing House Owl. 2004:851–89 (In Russ.). EDN: YLYRBH
2. Romanenko NA. Central venous catheter in oncohematological practice (Lecture and own data). Medicine: Theory and Practice. 2024;9(1):58–73 (In Russ.). https://doi.org/10.56871/MTP.2024.70.59.009
3. Sugak AB, Shchukin VV, Konstantinova AN, Feoktistova EV. Complications of central venous catheters insertion and exploitation// Issues of Hematology/Oncology and Immunopathology in Pediatrics. 2019;18(1):127–39 (In Russ.). https://doi.org/10.24287/1726-1708-2019-18-1-127-139
4. Sumin SA, Kuzkov VV, Gorbachev VI, Shapovalov KG. Catheteri zation of the subclavian and other central veins. Guidelines. Bulletin of Intensive Care named after. AI Saltanova. 2020;1:7–18 (In Russ.). https://doi.org/10.21320/1818-474X-2020-1-7-18
5. Klinicheskie rekomendacii. Anesteziologiya-reanimatologiya. Moscow: GEOTAR-Media, 2016:914–7 (In Russ.). EDN: XGHJDL
6. Olkhova lV, Popov VE. Spontaneous Catheter Separation from the Implanted Venous Port and Its Migration to the Venous Heart: Clinical Case. Onkopediatriya. 2018;5(2):127–32 (In Russ.). https://doi.org/10.15690/onco.v5i2.1915
7. Orlova OA, Semenenko TA, Akimkin VG, Yumtsunova NA. Clinical and epidemiological characteristics of catheter-associated bloodstream infections in patients with hematological profile. Medicinskiy Alphabit. 2020;(34):9–12 (In Russ.). https://doi.org/10.33667/2078-5631-2020-34-9-12
8. Greene ES. Challenges in reducing the risk of infection when accessing vascular catheters. J Hosp Infect. 2021;113:130–44. https://doi.org/10.1016/j.jhin.2021.03.005
9. Rickard CM, Flynn J, Larsen E, Mihala G, Playford G, Shaw J, et al. Needleless connector decontamination for prevention of central venous access device infection: a pilot randomized controlled trial. Am J Infect Control. 2021;49:269–73. https://doi.org/10.1016/j.ajic.2020.07.026
10. Rowley S, Clare S. Standardizing the critical clinical competency of aseptic, sterile, and clean technique with a single international standard. Aseptic Non Touch Technique (ANTT). J Assoc Vasc Access. 2019;24(4):12–7. https://doi.org/10.2309/j.java.2019.004.003
11. Smolyar AN, Ginzburg LM, Smirnov MA. Totally implantable central venous port: analysis of complications and their prevention. Pirogov Journal of Surgary, 2019;(12):13–7 (In Russ.). https://doi.org/10.17116/hirurgia201912113
12. Di Carlo I, Pulvireni E, Mannino M, Toro A. Increased use of percutaneous technique for totally implantable venous access devices. Is it real progress? A 27-year comprehensive review on early complications. Ann. Surg. Oncol. 2010;17(6):1649–56. https://doi.org/10.1245/s10434-010-1005-4
13. Romanenko NA. Central vein catheterization in oncohematology patients: complication, associated with procedure and care. HemaSphere. 2022;6(3):1487–8. https://doi.org/10.1097/01.HS9.0000849280.98974.58
14. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proc. 2006;81(9):1159–71. https://doi.org/10.4065/81.9.1159
About the Author
N. A. RomanenkoRussian Federation
Saint-Petersburg
Supplementary files
Review
For citations:
Romanenko N.A. Study of complications associated with central vein catheterization in patients with blood disorder. Extreme Medicine. 2024;26(3):106-112. https://doi.org/10.47183/mes.2024-26-3-106-112

















