Scroll to:
Effect of bovine prostate extract on the contractile activity of lymphatic vessels in rats
https://doi.org/10.47183/mes.2024-26-3-87-91
Abstract
Introduction. A comprehensive approach to treatment of chronic prostatitis, representing a widespread and poorly treatable disease, includes the use of antibacterial, anti-inflammatory medicines, etc. In this context, a promising approach to the treatment of chronic prostatitis involves the use of bioregulatory peptides isolated from bovine prostate tissue.
Objective. To study the effect of cattle prostate extract containing bioregulatory peptides on the functional activity of lymphatic vessels.
Маterials and methods. The study was performed on isolated lymphatic vessels of rats. The range of studied concentrations of the substance was 2–10 µg/ mL (in terms of water-soluble peptides).
Results. Bioregulatory peptides included into the prostate extract impacts the vasomotor activity of lymphatic vessels. In the range of studied concentrations (2–10 µg/ml), the substance has a stimulating effect on lymphangion motility. This is realized by increasing the rate of lymphatic vessel contractions, which effect is most pronounced at the concentration of 5 µg/ml as a 37.6% (p ≤ 0.05) of base level. The obtained stimulating effect is stable during 30-min “washing” of lymphatic vessels with physiological solution.
Conclusions. Water-soluble bioregulatory peptides contained in the extract of cattle prostate and having organotropic action on the prostate gland may contribute to the reduction of tissue edema by activating the motility of pelvic lymphatic vessels.
For citations:
Nechaykina O.V., Laptev D.S., Petunov S.G., Bobkov D.V., Kudryavtseva T.A. Effect of bovine prostate extract on the contractile activity of lymphatic vessels in rats. Extreme Medicine. 2024;26(3):87-91. https://doi.org/10.47183/mes.2024-26-3-87-91
INTRODUCTION
Chronic prostatitis is one of the most common and at the same time among the most understudied and poorly treated diseases. The main target patient population is predominantly of reproductive age. The course of chronic prostatitis is often complicated by impaired copulatory and generative functions [1]. Recently, the disease is increasingly detected in elderly men, sometimes in combination with benign prostatic hyperplasia [2]. Thus, in men under 50 years of age, the incidence of confirmed prostatitis is only twice as high as in patients over 50 years of age [3].
The prostate gland has a well-developed intra-organ vasculature, including blood and lymphatic capillaries and postcapillaries. Blood and lymphatic microvessel beds form microvascular-muscular complexes with bundles of smooth myocytes in the anterior parts of the prostate gland and microvascular-glandular complexes with prostatic glands in the posterior and lateral parts of the prostate gland [4]. The leading place in the pathogenesis of chronic prostatitis is occupied by the disruption of lymphatic outflow into the vascular bed and congestion in glandular structures.
Due to the polyetiological and multifactorial nature of pathogenesis, the treatment of chronic prostatitis implies a comprehensive approach, including antibacterial and anti-inflammatory therapies, physiotherapeutic procedures, and other effects. A promising approach in the treatment of chronic prostatitis involves the use of bioregulatory peptides isolated from bovine prostate tissue [2]. The biological effect of prostatic peptides isolated from the bovine prostate gland was first revealed in the mid-1980s. Under experimental conditions it was revealed that polypeptides from the prostate gland possessing tropism to blood vessels increase the antiaggregatory activity of vascular walls [5]. Prostatic peptides, which along with other regulatory peptides are characterized by a lack of species specificity, also have a pronounced tropism to prostate tissues, permitting their consideration as a target organ.
The pharmaceutical market offers a large number of medicines having bovine prostate extract as an active ingredient. Active pharmaceutical substances included in the State Register of Medicinal Products are used for the production of these medicines. However, the features of their composition and strength of their biological effect are significantly determined by their widely varying production technology [6]. Substance-mixture Prostatilen® produced by AO MBNPK “Cytomed” obtained by tangential ultrafiltration technology is an active pharmaceutical substance used in the manufacture of the peptide drug Prostatilen®.
One of the formulations of Prostatilen® is rectal suppository. Its active ingredient is a complex of water-soluble peptides obtained from the prostate tissue of sexually mature bulls with addition of glycine. The ability of Prostatilen® to restore blood rheological properties and improve microcirculation in target organs leads to hemodynamic normalization and provides treatment of inflammatory diseases of the prostate gland [7]. The high bioavailability of Prostatilen® is provided by the low molecular weight of its peptide fraction components and realized by passive transport of drug through enterocytes of rectal mucosa. Peptides are absorbed by the rectal vessels and exert their effect through the system of anastomoses on the pelvic organs, including the prostate gland located in anatomical proximity to the injection site [8]. The outflow of interstitial fluid containing regulatory peptides is ensured by adequate contractile activity of lymphatic vessels sensitive to the action of various vasoactive substances, including endogenous modulators of functional state [9–11].
Isolated lymphatic vessels with spontaneous rhythmic contractile activity are the one of available and informative experimental models used to study the effect of vasoactive substances on the transport function of lymphatic system. In this regard, the evaluation of the peptide complex (active substance of Prostatilen® drug) effect on the contractile activity of lymphatic vessels is an urgent problem, whose solution can be used in the development and optimization of therapeutic methods for treating chronic prostatitis.
The aim of the study was to investigate the effect of the substance-mixture Prostatilen® (hereinafter referred to as Prostatilen®) on the functional activity of lymphatic vessels as a potential target of the therapeutic effect of the studied extract.
МАTERIALS AND METHODS
The study was performed on isolated segments of the anterior mesenteric lymphatic duct of sexually mature male nonlinear white rats weighing 250–300 g. The samples were obtained from the branch of SIC “Kurchatov Institute” PIAR — “Nursery of laboratory animals ‘Rappolovo’ (Leningrad region) — PIAR — “Rappolovo Laboratory Animal Nursery” (Leningrad Region). The maintenance and feeding of laboratory animals were performed in accordance with GOST 33215-2014 “Guidelines for the maintenance and care of laboratory animals” from 2016. Healthy sexually mature animals that underwent a 14-day quarantine were used for the studies. Microclimate parameters (temperature, relative humidity, air exchange rate parameters), as well as the quality of feed and bedding material were controlled in the vivarium premises. The animals were fed a standard diet in the form of pelleted feed. In the rooms where experimental animals were kept, a lighting regime of 12 h day/12 h night was established.
The experimental study was conducted in accordance with the European Convention for the Protection of Animals Used in Experimentation (Directive 86/609/EEC) and approved by the Bioethics Commission (protocol #5 of 16.12.2022).
The experimental study of the Prostatilen® effect on the functional parameters of isolated lymphatic vessels of rats ex vivo was carried out using a multi-channel myograph Multi Wire Myograph System DMT 620M (DMT, Denmark) according to the previously described method [12]. The parameters of contractile activity of lymphatic vessels, including the level of tonic tension, amplitude and phasic contraction rate (CR), were recorded using a PowerLab Data acquisition system 8/30 (ADInstruments, USA) with subsequent processing in the LabChartProUpgrade 7.0 software package.
The test object is a mixture-substance Prostatilen®, as well as a Prostatilen® solution with a content of 11.7% water-soluble peptides (according to the manufacturer’s passport). Prostatilen® was used in concentrations (based on the content of water-soluble peptides) of 1 µg/mL, 2 µg/mL, 5 µg/mL, and 10 µg/mL was added to perfusate to research the reactivity of lymphatic vessels. The exposure time of each concentration was 20 min. A Krebs-Henseleit saline solution with the same exposure time was used as a control.
Statistical processing of the data was carried out using methods of descriptive and analytical statistics using the GraphPad Prism 5.04 software package. The critical level of significance was taken to be p≤0.05. The median value was used to describe the central tendency; the first (Q1) and third (Q3) quartiles were used as a measure of dispersion. Data were analyzed using the Wilcoxon T-test for related samples. The Mann-Whitney U-test was used to detect intergroup differences.
RESULTS
Like any biological object, the parameters of lymphatic vessels phase activity are characterized by a certain variability (Table 1). Therefore, when analyzing the obtained results, we used relative units characterizing the change of parameters under the action of the drug compared to the base values, which were taken as 100%. When performing the work, data collection was carried out in parallel (simultaneously) in all experimental groups.
The results of experiments evaluating the effect of Prostatilen® on isolated lymphatic vessels are summarized in Table 2.
It was found that Prostatilen® application in the minimum studied concentration (1 µg/mL) during 20-minute period had no effect on the contractile activity of lymphangions. At a 20-minute exposure with concentrations of 2 µg/mL, 5 µg/mL and 10 µg/mL, Prostatilen® caused an increase in CR from background values by 23.7% (p≤0.05), 37.6% (p≤0.05) and 26.8% (p≤0.05), respectively. In all studied concentrations, the contraction amplitude and tonus of lymphatic vessels under the influence of substance-mixture were registered at the level of background values and did not differ statistically significantly from the control.
In addition to bioregulatory peptides, the composition of Prostatilen® includes the amino acid glycine. Being an integral part of the mitochondrial respiratory chain, glycine is actively involved in the process of cell renewal and oxygenation, in the synthesis of proteins (in particular, glutathione tripeptide), and in detoxification reactions. It has a wide range of anti-inflammatory, cytoprotective and immunomodulatory properties [13]. According to the literature, glycine also has an effect on the blood and lymphatic vessels that make up the cardiovascular system [14–16]. In order to exclude the intrinsic effect of glycine on mesenteric lymphatic vessels, experiments were conducted with Prostatilen® solution used for the manufacture of the liquid formulation of Prostatilen® medicine (solution for intramuscular administration) and not containing glycine. A minimum effective concentration of 2 µg/mL (based on the content of water-soluble peptides) was selected.
As follows from the data shown in Table 2, a Prostatilen® solution at a concentration of 2 µg/mL resulted in a 29.6% (p≤0.05) increase in CR of lymphangions as compared to background. At the same time the amplitude of contractions and tonic tension were registered at the level of background values. The obtained results are comparable with the results obtained when studying the effect of Prostatilen® substance-mixture on lymphatic vessels, which demonstrates the mechanism of the chronotropic effect of Prostatilen® due to the action of bioregulatory peptides.
Table 1. Background indices of contractile activity of rat lymphatic vessels in the experimental groups. Absolute data are presented as Me (0.25; 0.75)
|
Indicators |
Saline solution, n = 12 |
Substance-mixture Prostatilen® |
|||
|
1 μg/mL, n = 11 |
2 μg/mL, n = 11 |
5 μg/mL, n = 11 |
10 μg/mL, n = 13 |
||
|
CR, min-1 |
4.55 (3.28; 6.95) |
3.00 (2.60; 6.25) |
5.70 (2.35; 6.65) |
5.20 (2.85; 7.10) |
5.60 (4.15; 8.50) |
|
Amplitude, mN |
0.62 (0.49; 0.83) |
0.45 (0.34; 0.70) |
0.80 (0.31; 0.90) |
0.73 (0.42; 1.03) |
0.65 (0.40; 0.97) |
|
Tonus, mN |
1.38 (1.29; 0.75) |
1.69 (1.38; 2.15) |
1.72 (1.16; 2.15) |
1.82 (1.55; 2.54) |
1.49 (1.11; 1.76) |
Table prepared by the authors using their own data
Table 2. Parameters of contractile activity of rat lymphatic vessels under the influence of Prostatilen®. Relative data in percentages are presented as Me (0.25; 0.75)
|
Investigated substance |
n |
FC |
Amplitude |
Tonus |
|
Saline solution |
12 |
103.6 (98.5; 109.1) |
100.6 (96.9; 104.8) |
98.5 (97.5; 100.4) |
|
substance-mixture Prostatilen®, 1 μg/mL |
11 |
106.6 (101.7; 108.7) |
104.0 (94.6; 106.6 |
99.3 (97.9; 103.9) |
|
substance-mixture Prostatilen®, 2 μg/mL |
11 |
123.7 (111.9; 130.6)* |
100.4 (97.7; 104.5) |
99.8 (97.2; 101.7) |
|
substance-mixture Prostatilen®, 5 μg/mL |
11 |
137.6 (129.9; 158.3)* |
99.0 (94.0; 101.8) |
99.9 (97.4; 100.9) |
|
substance-mixture Prostatilen®, 10 μg/mL |
13 |
126.8 (114.7; 129.8)* |
97.6 (94.6; 100.7) |
100.7 (99.1; 104.7) |
|
solution of Prostatilen® 2 μg/mL (without glycine) |
12 |
129.6 (112.8; 144.0)* |
100.8 (97.9; 107.7) |
99.6 (97.8; 101.0) |
Table prepared by the authors using their own data
Note: * — statistically significant difference from background values at p < 0.05
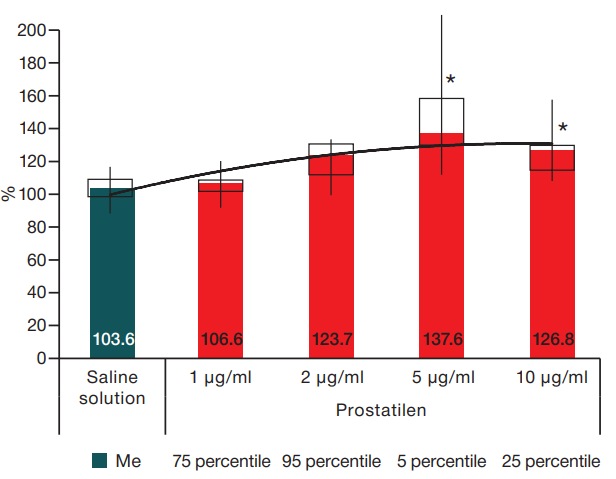
Figure prepared by the authors using their own data
Fig. 1. Changes in the rate of lymphatic vessels contractions when exposed to Prostatilen®. Exposure duration 20 min. Data presented as a percentage relative to the background in the form of Me. * — statistically significant difference from saline solution at p < 0.05.
RESULTS AND DISCUSSION
Initial changes in the phasic contractions rate occurred as an effect of substance-mixture Prostatilen® at a concentration of 2 µg/mL. The effect increased with increasing concentration of Prostatilen®. The maximum increase in CR was observed as effect of Prostatilen® in concentration of 5 µg/mL. When the concentration of the substance was increased up to 10 kg/mL, the positive chronotropic effect on lymphatic vessels was maintained. Although the increase in CR was slightly lower than at the previous concentration, this increase was also statistically significantly different from the control (p≤0.05). Thus, Prostatilen® has a positive chronotropic effect on lymphatic vessels. Dynamics of changes in the CR of lymphangions under Prostatilen® exposure in all concentrations is presented in Figure 1.
CR is well known to be associated with the spontaneous generation frequency of action potentials in smooth muscle pacemaker cells where the basis for the occurrence of action potentials is spontaneous short-term depolarization (Spontaneous Transitory Depolarization), which occurs due to the opening of Ca2+-dependent Cl-channels during intracellular release of Ca2+ ions from inositol-1,4,5-triphosphate-sensitive depot [17]. It can be assumed that Prostatilen® promotes the release of Ca2+ ions from intracellular depot, which, in turn, leads to increased CR.
To study the stability of the obtained stimulating effect of Prostatilen®, we analyzed the change of CR during a “washing” period, when the working chamber of the myograph was perfused with Krebs-Henseleit solution without the active substance. After a 30-min washout period after the expose of Prostatilen® at a concentration of 10 µg/mL, the phasic contractile activity exceeded the background values by 30.1% (p≤0.05) indicating the prolonged action of the drug. Such an effect of the aftereffect of bioregulatory peptides can be explained in terms of I.P. Ashmarin’s theory of the functional and regulatory continuum of the system of regulatory peptides [18]. Intracellular processes following peptide receptions are associated with the action of the latter on secondary messenger systems, which in turn trigger an intracellular cascade of sequential enzyme activation that ultimately manifests itself as a change in metabolic processes in the cell and determines the physiological response. It is fundamentally important that bioregulatory peptides are capable of triggering a number of processes at all levels of the metabolic hierarchy of cells following interaction with a receptor — from the membrane to the genome — with different durations — from minutes (or fractions thereof) to hours. This makes possible the existence of cascade effects in the organism caused by the introduction or release of a particular bioregulatory peptide [19].
CONCLUSIONS
- As a result of this study, it has been shown for the first time that the substance-mixture Prostatilen® demonstrates a vasoactive effect on lymphatic vessels. Across the concentration range between 2 and 10 µg/mL, Prostatilen® stimulates the motility of lymphangions, thus providing a basis to consider isolated lymphatic vessels as an adequate physiological model for the study of vasoactive properties of the medicine.
- Water-soluble bioregulatory peptides included in the composition of the Prostatilen® medicine can be assumed not only to have a directed (organotropic) effect on the prostate gland and organs of the urogenital system, but also making a contribution to the reduction of tissue edema by activating the motility of lymphatic vessels of the pelvic organs, once again confirming the feasibility of regulatory peptides in the treatment of diseases associated with inflammatory and congestive phenomena.
References
1. Beliy LE. Prostatic peptides for the correction of pathospermia in patients with chronic bacterial prostatitis. Meditsinsky Sovet. 2018;21:178–82 (In Russ.). https://doi.org/10.21518/2079-701X-2018-21-178-182
2. Gorilovsky LM., Dobrokhotov MM. Chronic prostatitis. Medical advice. 2010;(7–8):72–7 (In Russ.). EDN: MVYXVD
3. Uchvatkin GV, Tatarintseva MB. Prostatic peptides in the treatment of prostate diseases. Urological reports. 2017;7(3):44–8 (In Russ.). https://doi.org/10.17816/uroved7344-48
4. Petrenko VM. Vascular bed of the prostate. International Journal of Experimental Education. 2010;7:48–9 (In Russ.). EDN: RAJOOF
5. Khavinson VH., Morozov VG., Kuznik BI, et al. Effect of polypeptides from the prostate on the hemostasis system. Pharmacology and toxicology. 1985;48(5):69–71 (In Russ.). EDN: OXGGLQ
6. Savel’ev SA., Dorosh MY., Lagereva IA, et al. Influence of manufacturing technology on the properties of active pharmaceutical ingredients of prostate extract. Pharmaceutical Chemistry Journal. 2022;56(3): 50–8 (In Russ.). https://doi.org/10.30906/0023-1134-2022-56-3-50-58
7. Kuzmin IV, Borovets SYU, Gorbachev AG, Al-Shukri SKH. Prostatic bioregulatory polypeptide prostatilen: pharmacological properties and 30-year experience of clinical application in urology. Urology Reports. 2020;10(3):243–58 (In Russ.). https://doi.org/10.17816/uroved42472
8. https://cytomed.ru/wp-content/uploads/2023/11/ohlp_prostatilen-3-mg.pdf
9. Razavi MS, Dixon JB, Gleason RL. Characterization of rattail lymphatic contractility and biomechanics: incorporating nitric oxide-mediated vasoregulation. JR Soc Interface. 2020;17(170):20200598. https://doi.org/10.1098/rsif.2020.0598
10. Johnston MG., Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293:294–7. https://doi.org/10.1038/293294a0
11. Lobov GI, Unt DV. Glucocorticoids stimulate the contractile activity of lymphatic vessels and lymph nodes. Regional blood circulation and microcirculation. 2017;4(64):73–9 (In Russ.). https://doi.org/10.24884/1682-6655-2017-16-4-73-79
12. Nechaykina OV, Laptev DS, et al. Effect of Unsymmetrical Dimethylhydrazine on Isolated Heart and Lymphatic Vessels. Bull Exp Biol Med. 2021;172(9): 283–6 (In Russ.). https://doi.org/10.1007/s10517-022-05380-y
13. Perez-Torres I. Zuniga-Munoz A. M., Guarner-Lans V. Beneficial Effects of the Amino Acid Glycine. Mini Rev Med Chem. 2017;17(1);15–32. https://doi.org/10.2174/1389557516666160609081602
14. Stalberg HP, Hahn RG, Hjelmqvist H, Ullman J, Rundgren M, Stalberg HP. Haemodynamics and fluid balance after intravenous infusion of 1.5% glycine in sheep. Acta Anaesthesiol Scand. 1993;37(3):281–7. https://doi.org/10.1111/j.1399-6576.1993.tb03716.x
15. Podoprigora GI, Nartsissov YR. Effect of glycine on the microcirculation in rat mesenteric vessels. Bull Exp Biol Med. 2009;147(3):308–11. https://doi.org/10.1007/s10517-009-0498-y
16. Sawada M, McAdoo DJ, Ichinose M, Price CH. Influences of glycine and neuron R14 on contraction of the anterior aorta of Aplysia. Jpn J Physiol. 1984;34(4):747–67. https://doi.org/10.2170/jjphysiol.34.747
17. Von der Weid PY, Rahman M, et al. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: Pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295:1989–2000. https://doi.org/10.1152/ajpheart.00007.2008
18. Ashmarin IP, Obukhova MF. Sovremennoe sostoianie gipotezy o funktsional’nom kontinuume reguliatornykh peptidov [Current state of the hypothesis on functional continuum of regulatory peptides]. Vestn Ross Akad Med Nauk. 1994;10:28–34 (In Russ.). PMID: 7534535.
19. Ashmarin IP, Obukhova MF. Regulatory peptides: functionally continuous integrity. Biochemistry. 1986;51(4):531–45 (In Russ.).
About the Authors
O. V. NechaykinaRussian Federation
Leningrad Region
D. S. Laptev
Russian Federation
Leningrad Region
S. G. Petunov
Russian Federation
Leningrad Region
D. V. Bobkov
Russian Federation
Leningrad Region
T. A. Kudryavtseva
Russian Federation
Saint-Petersburg
Supplementary files
Review
For citations:
Nechaykina O.V., Laptev D.S., Petunov S.G., Bobkov D.V., Kudryavtseva T.A. Effect of bovine prostate extract on the contractile activity of lymphatic vessels in rats. Extreme Medicine. 2024;26(3):87-91. https://doi.org/10.47183/mes.2024-26-3-87-91
















