Scroll to:
General toxic effect of a chitosan-based hemostatic agent implanted in rats
https://doi.org/10.47183/mes.2024-26-4-49-57
Abstract
Introduction. Hemostasis of ongoing bleeding during cavitary surgical interventions is an urgent problem both in civil and military healthcare. The development of new effective and affordable agents for hemostasis of internal bleeding and their introduction into clinical practice may contribute to increasing the survival of injured patients.
Objective. Study of general and local toxic effects of a local hemostatic agent (LHA) for intracavitary application.
Materials and methods. The study was performed on 20 mongrel rats (10 males and 10 females) weighing 180–220 g. The experimental group of animals was implanted with the LHA into the abdominal cavity at a dose of 512 mg/kg body weight (bw). The animals of the control group underwent surgery without LHA implantation. Data analysis was performed using the Microsoft Excel 2013 and Statistica 10.0 software applications.
Results. The health status, body weight, food and water consumption, and mass coefficients of internal organs in the experimental animals did not differ from those in the control group. The hematological and biochemical blood parameters showed values within the reference norm. The macro- and microscopic examination of the internal organs revealed a local irritant effect of the agent under study.
Conclusion. The laboratory animals tolerated the intraperitoneal implantation of the tested local hemostatic agent at a dose of 512 mg/kg bw well. A further study of its toxic properties and effectiveness is validated.
For citations:
Nosov A.M., Bondarenko A.A., Katretskaya G.G., Golovko K.P., Shultz A.V., Volkova M.V., Zolotoverkhaja E.A., Kubarskaya L.G., Bazhanova E.D., Gaikova O.N. General toxic effect of a chitosan-based hemostatic agent implanted in rats. Extreme Medicine. 2024;26(4):49-57. https://doi.org/10.47183/mes.2024-26-4-49-57
INTRODUCTION
Meticulous hemostasis during surgical interventions remains a pressing surgical issue in the 21st century. This problem is particularly significant for wounded and polytrauma patients, in whom timely hemostasis affects not only the timing of treatment, but also determines the overall outcome. The statistics of such injuries is contributed by road traffic accidents, negative crime situation, terrorist attacks, and military conflicts. Thus, a study by Trukhan et al. [1] found that during terrorist attacks in St Petersburg and Minsk, fatal injuries as the immediate cause of death were registered only in 33.3% of cases, while irreversible blood loss was the immediate cause of death in 66.7% of the deceased. The most frequent (almost 87.5%) cause of death in the victims was internal bleeding. In the structure of deaths from injuries received during armed conflicts, 78.1% of cases accounted for ongoing hemorrhage, with more than a half of the cases resulting from internal bleeding [2–6].
Although internal bleeding is almost impossible to stop at the pre-hospital stage, even timely patient transport to the operating room requires immediate control of bleeding. In addition to traumatic shock and hemorrhage, a cascade of pathophysiological reactions develops, including hypothermia, hypocoagulation, and acidosis (lethal triad). Early stopping of bleeding is a prerequisite for successful treatment of the wounded not only in the acute period of traumatic shock, but also in the third period of traumatic disease (development of infectious complications) [1]. At the same time, technical difficulties in intraoperative stopping of bleeding arise not only in urgent surgery (trauma and wounds), but also in elective surgery, particularly in patients with concomitant coagulopathy or those taking antiplatelet drugs and anticoagulants [7–10].
Various methods are currently used to stop hemorrhages occurring during cavitary surgeries, including wound closure, tight tamponade, electrocoagulation of various types, and local hemostatic agents in some cases (LHA) [11]. For intracavitary application, LHAs based on fibrinogen, cellulose, collagen, fibrin, thrombin, plant extracts, mucopolysaccharides (starches), etc., can be used [12].
Due to its wide availability and biocompatibility, chitosan is a promising substance for the preparation of LHAs. Materials based on chitosan and/or modified chitosan (suspensions, sponges, bandages, etc.) were found to be effective for stopping bleeding in vitro, promoting coagulation by activating platelets and/or agglutinating erythrocytes [13–17]. Previously, we demonstrated the high efficacy of a chitosan-based LHA in a model of intensive intra-abdominal bleeding in large biological objects [18].
To date, the action of chitosan and its derivatives has been extensively studied. However, before introduction into clinical practice, each newly developed polymer should be studied not only in terms of its efficacy, but also with regard to its safety for the potential patient due to the wide variability of formulation components. In this context, safety refers to both biocompatibility with the morphological structures in the area of direct contact and general toxicity to the organism. Such studies are also important due to the possible residue of the material in the human body.
In this study, we set out to investigate the general and local toxic effects of a hemostatic agent based on 90% lactic acid chitosan, 5% glycerol, and 5% calcium chloride for intracavitary application.
МАТЕRIALS AND METHODS
The study was carried out using the facilities of Golikov Research Center of Toxicology (Saint Petersburg, Russia). In total, 20 mongrel rats (10 males and 10 females) weighing 180–220 g provided by the Rappolovo Laboratory for Animal Nursery of the National Research Center “Kurchatov Institute” were divided into experimental and control groups. The experimental group included male and female animals which were implanted with the local hemostatic agent (LHA) under study. The control group included male and female falsely operated animals. Animals were assigned to groups by sex and using the body weight as the main criterion such that the individual body weight value did not deviate from the mean value within the same sex by more than 10%. Prior to the study, all animals were adapted to the housing conditions for five days. During this period, the general condition of the rats was monitored daily by visual inspection.
The dose of LHA to be administered to animals was calculated based on the dose used in an efficacy study, 90 mg/kg (per finished dosage form, FDF) [18]. Considering the metabolic coefficient, the value of LHA dose for rats was: 90×37 (metabolic coefficient for a 60 kg human)/6.5 (metabolic coefficient for a 200 g rat) ≈ 512 mg/kg [19]. Thus, the investigated dose of the study sample administered to rats was 512 mg/kg bw. The mass of implanted LHA and, accordingly, the drug dose for each animal was calculated based on the value of its weight. Sterile samples (radiation sterilization) of LHA were used in the study. LHA were implanted into the abdominal cavity of rats in native form. LHA are plates of light-yellow color with a relief surface and a porous structure, 4–6 mm thick. The appearance of the product is presented in Fig. 1.

Figure prepared by the authors
Fig. 1. Appearance of the implemented local hemostatic agent (LHA)
The animals in the control group underwent surgical intervention without implantation of the test sample (falsely operated animals).
Injectable general anesthetic tiletamine/zolazepam (Zoletil® 100) was used as anesthesia during LHA implantation. This medication has a good analgesic and sedative effect. The medication was administered intraperitoneally at a dose of 50 mg/kg bw; complete relaxation and absence of pain sensitivity occurred 10–15 min after drug administration. The average duration of anesthesia was 60–90 min.
The surgical field was prepared according to the generally accepted rules of aseptic and antiseptic surgery. Lidocaine solution for injection 20 mg/mL in the volume of 0.2 mL was injected subcutaneously into the incision area for anesthesia. During anesthesia, the eyes of the animals remained open and a Vidisik® eye gel was applied to prevent the development of dry keratoconjunctivitis.
Surgical procedure. To perform a midline laparotomy, the animal was in the dorsal position. The skin incision was performed along the midline from the umbilicus in caudal direction with the length of about 1 cm, then blunt dissection of tissues up to visualization of the white line of the abdomen was performed. The peritoneum was dissected with a scalpel, the edges of the abdominal wall were grasped with tweezers and lifted upwards for convenient visualization of the abdominal organs of the rat. It should be noted that LHA was applied in a dry form; the studied material was freely placed in the abdominal cavity (Fig. 2).
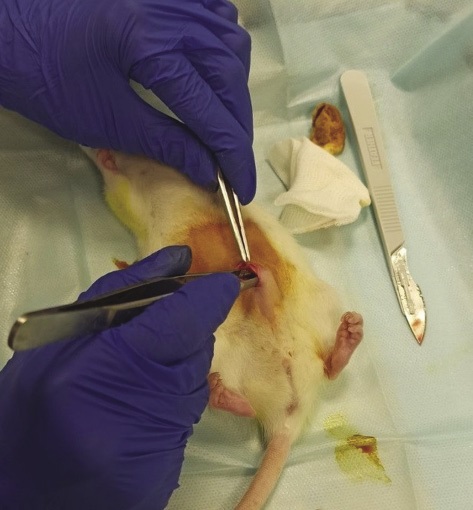
Figure prepared by the authors
Fig. 2. Implantation of the local hemostatic agent (LHA) into the abdominal cavity of a rat
Then the abdominal cavity was stitched layer by layer (Fig. 3). The experimental animals were placed in a heated cage with a warm floor for recovery after anesthesia. Full awakening of the animals occurred 2–3 h after induction of anesthesia.
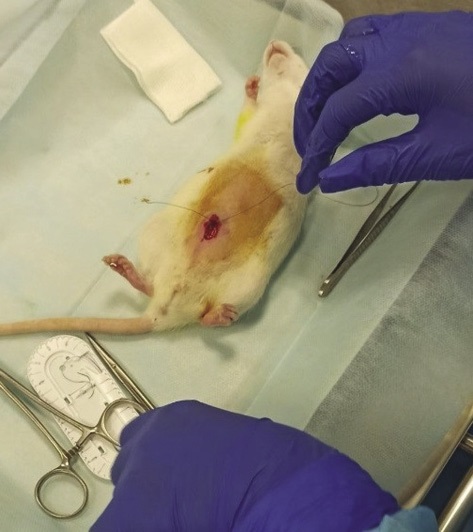
Figure prepared by the authors
Fig. 3. Suturing of the rat peritoneum after implantation of the local hemostatic agent
During the study, we evaluated the animal survival rate, clinical picture of intoxication, body weight dynamics, water and feed consumption, hematological and biochemical parameters. In addition, the macro- and microscopic examination of internal organs was carried out along with calculation of the mass ratios of organs and evaluation of local reactions after LHA implantation [20].
The general condition of the rats was evaluated daily; deviations in the consumption of food and water by animals in separate cages were noted. Body weight was monitored one day before LHA implantation and then weekly after introduction into the experiment. The body weight of the starved animal immediately before necropsy was taken to calculate the percentage of internal organ weight to animal body weight. Animals were deprived of food the night before necropsy. Access to water was not restricted.
Blood sampling for studying hematological and biochemical parameters was performed on the 29th day of the study by exsanguination of animals after inhalation of carbon dioxide (CO2).
Biological material was collected from animals after 14–15 h of fasting. Blood from rats in the amount of 0.5 ml was collected in blood collection tubes with ethylenediaminetetraacetic acid (EDTA). The blood sample was thoroughly mixed and placed in a refrigerator.
The sample was analyzed 120 min after collecting the biological material. For general clinical blood analysis, an automatic hematological analyzer Advia 2120 by Siemens (Germany) was used. The hematological analyzer is fully automated for counting blood cells and erythrocyte indices [21].
To assess biochemical parameters, blood was collected in a dry vacutainer. To obtain blood serum, whole blood samples were centrifuged at 3000 rpm at 4°C for 10 min followed by collection of the supernatant fluid (serum). Biochemical parameters (total protein, urea, creatinine, glucose, cholesterol, total bilirubin, alanine aminotransferase ALT, aspartate aminotransferase AST, alkaline phosphatase ALP) were determined on an A-25 biochemical analyzer from BioSystems (Spain) using Vector-Best JSC kits (Russia). The internal quality control of the studies was performed using control materials from Vector-Best JSC (Russia) [22].
Inhalation (CO2) followed by exsanguination was used as the euthanasia method. An Open Science euthanasia unit (Russia) was used for euthanasia [23].
On day 29 of the study, the animals were subjected to a complete necropsy, which included an examination of the external surface of the body, all passages, cranial, thoracic, abdominal cavities, and their contents. During the planned necropsy, the brain, heart, lungs (pair), liver, adrenal glands (pair), kidneys (pair), spleen, ovaries/testes (pair), and thymus were weighed in all the studied animals. Paired organs were weighed together. In addition to the absolute weight of the organs, the ratio of the organ weight to the body weight of the animal was calculated, presented in percentiles (‰). A histological analysis of the isolated organs and tissues was performed in the animals with an additional assessment of the local effect of LHA after its implantation (peritoneal histology). Tissue samples were collected, which were dehydrated and impregnated with paraffin. The prepared sections were stained with haematoxylin and eosin. Histological preparations were examined by light microscopy using a Leica DM1000 microscope (Germany) under ×100 magnification.
A mathematical and statistical analysis of the results was performed using the Microsoft Excel 2013 and Statistica 10.0 software packages. The Mann–Whitney test (U-test) was used to assess the differences between the two groups [24]. Descriptive statistics were presented as a measure of central tendency and dispersion indicators. The following descriptive statistics of quantitative indicators were calculated: median (Me), upper (UQ), and lower (LQ) quartiles. Differences in the indicators of the experimental animals in relation to the control were considered statistically significant at p ≤ 0.05.
RESULTS
After intraperitoneal implantation of the studied sample of LHA in rats at a dose of 512 mg/kg bw, no lethal outcomes were registered. The animals did not differ from the control group in appearance and behavior. The death of one male in the control group of falsely operated animals due to postoperative complications was registered. The clinical condition of the experimental animals was satisfactory during the entire period of observation. Signs of intoxication were not observed in any animal. Water and feed consumption in rats from experimental and control groups did not differ.
The effect of the investigated sample on the body weight of sexually mature animals was estimated by a comparative assessment of the body weight of rats after LHA implantation and falsely operated animals at several examination stages: base (one day before implantation of LHA), 1 week, 2 week, 3 week, and 4 week of the experiment.
The data on body weight of rats in the studied groups are presented in Figs. 4 and 5. It is important to note that the initial (base) body weight of both females and males did not differ significantly between the LHA-implanted and control groups.
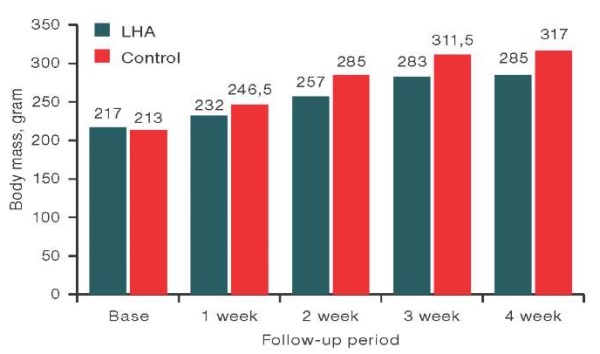
Figure prepared by the authors using their own data
Fig. 4. Body weight of male rats at different stages of examination
Note: data are presented as median values.
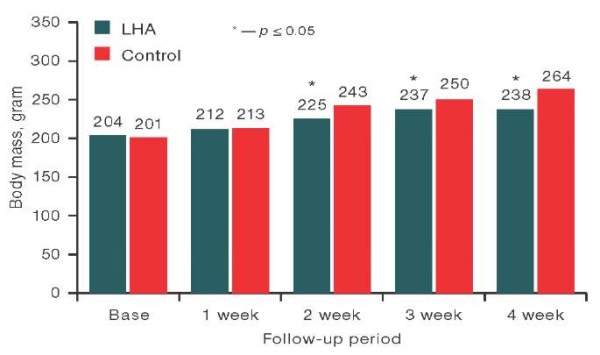
The figure has been prepared by the author using his own data
Fig. 5. Body weight of female rats at different stages of examination
Note: data are presented as median values.
The comparative assessment of body weight of male rats after implantation of the studied sample and the weight of animals from the control group in the observation period, base (day before implantation), 1 week, 2 week, 3 week, and 4 week, showed no significant differences between the studied groups using the Mann–Whitney test. At the same time, statistically significant differences in the body weight of female rats from the experimental group at 2 weeks of observation (p = 0.022), 3 weeks (p = 0.034), and 4 weeks (p = 0.012) compared to the control were revealed. An analysis of the data presented in Fig. 5, showed that the values of body weight of female rats from the experimental group were statistically significantly lower than those in the control group at 2 weeks by 7.4%, at 3 weeks by 5.2%, at 4 weeks by 9.9%, although the values did not exceed the reference values for this type of animals.
Table 1 shows the data of hematologic parameters in animals of the control and experimental groups. An analysis of the values of hematological parameters in male rats of the experimental group revealed a statistically significant increase in monocyte percentage by 18% and absolute monocyte count of 42% compared to the control group of animals. At the same time, the study and subsequent evaluation of the values of hematologic parameters in females in the experimental group compared to the control revealed a statistically significant decrease in hemoglobin concentration and mean corpuscular volume by 5% and 7%, respectively [25].
Table 1. Comparative evaluation of hematological parameters of male and female rats in experimental and control groups 29 days after the onset of the experiment
|
Indicator |
Control (♂) |
LHA, 512 mg/kg bw (♂) |
Control (♀) |
LHA, 512 mg/kg bw (♀) |
|
Number of animals in the group |
4 |
5 |
5 |
5 |
|
White blood cell count, 10³/μL |
9.68 [ 8.17; 11.7] |
10.84 [ 10.19; 12.71] |
7.51 [ 7.43; 8.16] |
7.99 [ 7.09; 8.94] |
|
Red blood cell count, 10⁶/µL |
7.58 [ 7.52; 8.2] |
7.58 [ 7.55; 7.91] |
7.36 [ 7.31; 7.37] |
7.42 [ 7.31; 7.63] |
|
Hemoglobin, g/dL |
14.95 [ 14.2; 15.95] |
14.80 [ 14.80; 15.00] |
14.30 [ 14.10; 14.50] |
13.60* [ 13.0; 13.8] |
|
Hematocrit, % |
42.25 [ 41.25; 46.0] |
42.50 [ 42.40; 43.20] |
41.90 [ 41.40; 42.20] |
40.30 [ 39.0; 41.2] |
|
Mean corpuscular volume, fL |
55.20 [ 54.5; 56.65] |
55.90 [ 54.6; 56.4] |
57.20 [ 57.0; 57.3] |
53.40* [ 52.60; 55.6] |
|
Platelet count, 10³/μL |
787.00 [ 686; 928] |
826.00 [ 601; 978] |
983.00 [ 962; 1022] |
970.00 [ 946.0; 1092.0] |
|
Mean platelet volume, fL |
6.85 [ 6.55; 7.75] |
6.90 [ 6.70; 7.10] |
7.10 [ 7.0; 7.20] |
7.20 [ 7.10; 7.30] |
|
Neutrophil % |
28.20 [ 20.75; 38.25] |
25.80 [ 23.20; 25.90] |
21.10 [ 20.50; 32.40] |
35.50 [ 28.80; 47.40] |
|
Lymphocyte % |
66.95 [ 56.0; 72.85] |
66.60 [ 65.30; 68,20] |
70.70 [ 61.40; 74.90] |
59.40 [ 46.40; 65.10] |
|
Monocyte % |
3.45 [ 2.70; 3.65] |
4.20* [ 4.10; 4.50] |
2.80 [ 2.70; 3.00] |
2.70 [ 2.10; 2.70] |
|
Eosinophil % |
0.85 [ 0.75; 1.60] |
0.80 [ 0.80; 1.10] |
1.20 [ 1.20; 1.50] |
1.10 [ 0.90; 2.10] |
|
Basophil % |
0.90 [ 0.75; 1.20] |
1.00 [ 0.80; 1.20] |
0.70 [ 0.60; 1.80] |
0.40 [ 0.40; 0.50] |
|
Unidentified cells, % |
0.75 [ 0.60; 1.00] |
1.10 [ 1.00; 2.00] |
0.90 [ 0.90; 1.10] |
0.90 [ 0.60; 0.90] |
|
Abs. neutrophil count, 10³/μL |
3.22 [ 2.01; 3.86] |
2.64 [ 2.33; 2.80] |
2.06 [ 1.50; 2.54] |
3.25 [ 2.30; 3.55] |
|
Abs. lymphocyte count, 10³/μL |
6.82 [ 5.02; 7.80] |
7.08 [ 6.78; 7.28] |
5.48 [ 5.01; 5.74] |
4.15 [ 3.18; 5.35] |
|
Abs. monocyte count, 10³/μL |
0.30 [ 0.28; 0.33] |
0.52* [ 0.40; 0.55] |
0.21 [ 0.20; 0.24] |
0.19 [ 0.17; 0.25] |
|
Abs. eosinophil count, 10³/μL |
0.09 [ 0.08; 0.15] |
0.09 [ 0.07; 0.14] |
0.09 [ 0.09; 0.11] |
0.08 [ 0.08; 0.09] |
|
Abs. basophil count, 10³/μL |
0.10 [ 0.08; 0.11] |
0.11 [ 0.10; 0.11] |
0.05 [ 0.05; 0.18] |
0.03 [ 0.02; 0.05] |
|
Unidentified cells, 10³/μL |
0.07 [ 0.07; 0.09] |
0.14 [ 0.10; 0.19] |
0.08 [ 0.07; 0.09] |
0.06 [ 0.05; 0.07] |
Table prepared by the authors using their own data
Note: data are presented as Me [ LQ; UQ].
* — statistical significance of differences between control and experimental groups, p < 0.05.
Biochemical parameters were studied following 29 days after the onset of the experiment in control animals (males and females), as well as in animals of the experimental group with implanted LHA (Table 2). It was found that the group of male rats after LHA implantation showed a significant increase in the aspartate aminotransferase activity by 18% compared to the control group.
Table 2. Comparative evaluation of biochemical parameters of male and female rats of experimental and control groups 29 days after the onset of the experiment
|
Indicator |
Control (♂) |
LHA, 512 mg/kg bw (♂) |
Control (♀) |
LHA, 512 mg/kg bw (♀) |
|
Number of animals in the group |
4 |
5 |
5 |
5 |
|
Total protein, g/L |
81.4 [ 75.9; 87.5] |
74.5 [ 71.9; 77.0] |
77.8 [ 77.6; 85.9] |
80.4 [ 79.0; 81.0] |
|
Urea, mmol/L |
6.0 [ 5.3; 7.3] |
8.7 [ 7.6; 9.0] |
7.5 [ 7.0; 8.9] |
10.0 [ 8.1; 10.8] |
|
Creatinine, µmol/L |
54.0 [ 50.5; 62.5] |
55.0 [ 55.0; 55.0] |
50.0 [ 49.0; 52.0] |
64.0 [ 63.0; 70.0] |
|
Glucose, mmol/L |
8.8 [ 8.2; 9.9] |
9.2 [ 9.0; 9.4] |
10.5 [ 9.8; 10.7] |
9.8 [ 9.5; 9.9] |
|
Cholesterol, mmol/L |
2.5 [ 2.2; 3.0] |
2.6 [ 2.1; 2.8] |
2.8 [ 2.4; 3.1] |
2.3 [ 2.2; 2.7] |
|
Total bilirubin, µmol/L |
1.7 [ 1.4; 1.7] |
1.5 [ 1.3; 2.2] |
1.2 [ 0.9; 1.4] |
0.6 [ 0.2; 1.1] |
|
Aspartate aminotransferase (AST), U/L |
166.0 [ 148.6; 171.3] |
196.5* [ 195.9; 200.6] |
73.7 [ 54.4; 74.6] |
102.0 [ 73.3; 114.4] |
|
Alanine aminotransferase (ALT), U/L |
76.0 [ 66.5; 86.0] |
81.0 [ 79.0; 90.0] |
70.0 [ 69.0; 77.0] |
69.0 [ 65.0; 71.0] |
|
Alkaline Phosphatase, U/L |
349.0 [ 265.5; 428.5] |
297.0 [ 275.0; 315.0] |
188.0 [ 182.0;224.0] |
236.0 [ 210.0;283.0] |
Table prepared by the authors using their own data
Note: data are presented as Me [ LQ; UQ].
* — statistical significance of differences between control and experimental groups, p < 0.05.
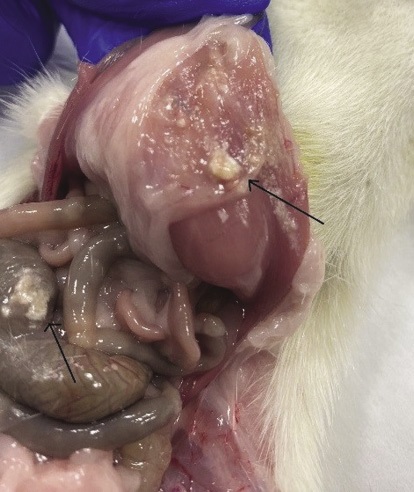
Figure prepared by the authors
Fig. 6. Fragments of the local hemostatic agent in the abdominal cavity of the rat
Note: fragments are indicated by arrows.
The control group of animals showed no changes in the peritoneal surface. In the experimental group, in one case, a fistula formation of the anterior abdominal wall was noted, the formation of which was caused by the reaction of foreign body rejection.
When examining the internal organs, thymus had a triangular shape, whitish in color and moderately dense in consistency. The size and shape of the heart were not changed. The surface of the lungs had a pale pink color; the lungs were collapsed at the opening of the chest. The spleen had a dark cherry color, smooth surface, and dense consistency. The stomach had the usual shape and size; the lumen was filled with dense food contents. The mucosa of the small and large intestine was shiny and smooth. The pancreas had a pale pink color and a lobular structure. The size and shape of the liver, kidneys, adrenal glands, testes, and ovaries remained unchanged. The brain substance had a moderate density; no ventricular dilatation was observed.
When evaluating the mass ratios of internal organs of male and female rats after implantation of LHA at a dose of 512 mg/kg bw and control animals using the Mann–Whitney test, no statistically significant differences were found between the studied groups.
A histological examination of the brain, heart, lungs, kidneys, thymus, testes, ovaries, adrenal glands, and spleen preparations of control animals and animals with LHA implantation in the abdominal cavity 29 days after the onset of the study revealed no significant differences between the groups. In the liver of animals of both groups, moderately or sharply expressed fatty dystrophy of hepatocytes was registered: in seven cases in the experimental group with LHA implantation and in six cases in control animals. At the same time, in one animal from the experimental group and in one animal from the control group, minor necroses with moderately expressed lympho-macrophage infiltration were registered. These changes could be caused by the influence of postoperative stress [26].
A histological examination of the peritoneum surface in the experimental group revealed fragments of LHA surrounded by a layer of lymphocytes and leukocytes and young fibrous connective tissue. Leuko-lymphocytic infiltration was also noted (Fig. 7), which are signs of local irritation. In the control group, an insignificant accumulation of lymphocytes and macrophages was registered.
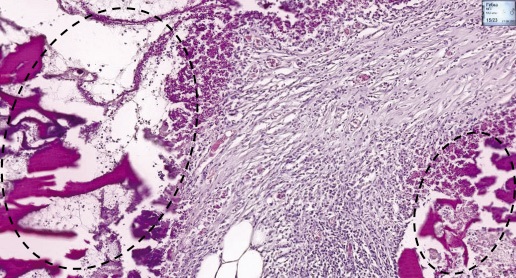
Figure prepared by the authors
Fig. 7. Histological section of rat peritoneum after implantation of local hemostatic agent
Note: on the peritoneal surface, fragments of LHA surrounded by a layer of lymphocytes and leukocytes and young fibrous connective tissue (dashed line). Haematoxylin and eosin staining. Magn. ×100.
DISCUSSION
The conducted study found that intraperitoneal implantation of the tested sample at a dose of 512 mg/kg bw does not lead to the death of animals or to the development of any signs of intoxication.
The animals in the experimental group did not differ from those in the control groups in terms of appearance and behavior. The general condition of the experimental animals was satisfactory throughout the entire experiment; the indices of water and feed consumption did not differ from the control. Body weight indices of the male rats did not differ from the control, while those of the female rats were statistically significantly lower compared to the control at several stages of examination. At the same time, the body weight of female animals within their group did not decrease throughout the study. Differences in the body weight of females of experimental and control groups could be related to the effect of postoperative stress.
When analyzing hematological parameters in animals during the experiment, a statistically significant increase in the proportion of monocytes and absolute number of monocytes in male rats of the experimental group compared to the control group was found, probably associated with the inflammatory reaction occurring in the abdominal cavity of rats.
In female rats with implanted LHA, a statistically significant decrease in hemoglobin concentration and mean corpuscular volume was observed compared to the control, apparently caused by the local irritant reaction of the peritoneum. The established increase in the aspartate aminotransferase activity in the group of male rats after LHA implantation compared to the control group is likely to be related to the damage to the animals’ liver due to postoperative stress [26].
It should be noted that the fluctuations of hematologic and biochemical parameters in animals did not exceed the reference intervals established for this type of animals [25].
The evaluation of the mass ratios of internal organs of rats after implantation of LHA at a dose of 512 mg/kg bw showed the absence of statistically significant differences between the studied groups. Morphologic macroscopic studies revealed no changes in the structure of internal organs in experimental animals; 29 days after the onset of the experiment, fragments of LHA were observed in the peritoneum and in the mesentery, which formed a dense conglomerate. According to the results of histological evaluation of the local action of LHA implantation, signs of a local irritating effect (compared to the control group of animals) were observed. The formation of a fistula of the anterior abdominal wall in one of the males of the control group suggests that the presented LHA samples should be removed after hemostasis is achieved.
CONCLUSION
The conducted study showed that intraperitoneal implantation of LHA at a dose of 512 mg/kg bw did not cause animal death or signs of intoxication. The body weight of female rats was statistically significantly lower than that of control rats at 2, 3, and 4 weeks of observation. However, the values did not exceed the reference values for this kind of animals.
An intraperitoneal implantation of LHA at a dose of 512 mg/kg bw to male rats, compared to the control, resulted in an increase in the absolute number of monocytes, as well as an increase in the activity of AST, not exceeding the reference values. This could be associated with the local irritating effect of the sample and/or postoperative stress.
The pathomorphological studies revealed no changes in the structure of internal organs of the animals. LHA fragments were found in the peritoneum and mesentery of rats, which formed a dense conglomerate. The histological evaluation of tissues showed signs of a local irritating effect.
The data obtained indicate normal tolerance of animals (rats) to implantation of the studied hemostatic agent in the abdominal cavity at a dose of 512 mg/kg bw, validating its further studies in terms of toxicity and specific activity.
Additional studies are needed to evaluate the biocompatibility and possible biodegradation time of the topical hemostatic agent. The study of biocompatibility and biodegradation of LHA based on chitosan should be carried out on larger laboratory animals (swine) with the purpose of subsequent extrapolation of the obtained results to humans.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows: Anastasiya A. Bondarenko — development of the research protocol, performance of the experimental part of the work, statistical processing of the obtained experimental data, Alena V. Shultz — performance of the experimental part of the study, Galina G. Katretskaya — scientific supervision, writing of the introduction section, Ekaterina A. Zolotoverkhaya, Larisa G. Kubarskaya — conducting biochemical and haematological studies of blood, Elena D. Bazhanova, Olga N. Gaikova — conducting histological study of organs, Artem M. Nosov, Konstantin P. Golovko, Marina V. Volkova — development of samples of local hemostatic agent, development of research protocol.
References
1. Trukhan AP, Samokhvalov IM, Tolmachev IA, Isakov VD, Golovko KP, Skakunova TYu, et al. The role of blood loss in the structure of thanatogenesis factors in explosive injury during peacetime. Bulletin of the Russian Military Medical Academy. 2020;2(70):66-9 (In Russ.). https://doi.org/10.17816/brmma50048
2. Samokhvalov IM, Goncharov AV, Chirskiy VS, Nosov AM, Golovko KP, Badmaev VB, et al. Potentially survivable casualties — reserve to reduce pre-hospital lethaility in injuries and traumas. Emergency medical care. 2019;20(3):10–7 (In Russ.). https://doi.org/10.24884/2072-6716-2019-0-3-10-17
3. Yanbarisova EV, Badretdinova YuA, Khasanov AG. Diagnosis and surgical tactics in injuries of parenchymatous organs of the abdominal cavity. Advanced in current natural sciences. 2014;6:73–6 (In Russ.). EDN: RYHWZJ
4. Chumakov AA, Malashenko VN, Khoreev AN, Kozlov SV. Indications for therapeutic dynamic videolaparoscopy for penetrating wounds of the abdominal cavity. Proceedings of the second scientific and practical conference of surgeons of the NorthWest and 23 Republic of Karelia together with St. Petersburg Research Institute of Emergency Aid. Prof. I.I. Dzhanelidze; Petrozavodsk; Republic of Karelia. 2000 (In Russ.)
5. Schoenfeld AJ. The combat experience of military surgical assets in Iraq and Afghanistan: a historical review. American journal of surgery. 2012;204(3):377–83. https://doi.org/10.1016/j.amjsurg.2011.09.028
6. Hoencamp R, Vermetten E, Tan E. Systematic review of the prevalence and characteristics of battle casualties from NATO coalition forces in Iraq and Afghanistan. Injury. 2014;45(7):1028–34. https://doi.org/10.1016/j.injury.2014.02.012
7. Atadzhanov Sh.K. Complications of laparoscopic cholecystectomy and its prevention. Journal of Theoretical and Clinical Medicine. 2005;2:89–93 (In Russ.). EDN: QEAGCB
8. Khadzhibaev AM, Malikov YuR, Atadzhanov ShK, Ermetov AT, Sametdinov NYu. Laparoscopic interventions in the diagnosis and treatment of postoperative intra-abdominal complications in urgent abdominal surgery. Annals of Surgical Hepatology. 2005;10(2):230 (In Russ.). EDN: HTZYQN
9. Shinkevich DS, Magilevets MV. Comparative evaluation of different methods of surgical hemostasis used during tooth extraction in hematologic patients. Materials of the national congress with international participation Parinsky Readings 2020. Topical issues of diagnostics, treatment and dispensary of patients with surgical pathology of maxillofacial region and neck; 2020 May 7–8; Minsk, Republic of Belarus. Minsk: Belarusian State University; 2020. р. 272–77 (In Russ.). EDN: MLCPRO
10. Kim JC, Choi SS, Wang SJ, Kim SG. Minor complications after mandibular third molar surgery: type, incidence, and possible prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102(2):4–11. https://doi.org/10.1016/j.tripleo.2005.10.050
11. Rago AP, Marini J, Duggan MJ, et al. Diagnosis and deployment of a self-expanding foam for abdominal exsanguination: Translational questions for human use. Journal of Trauma and Acute Care Surgery. 2015;78(3):607–13. https://doi.org/10.1097/TA.0000000000000558
12. Belozerskaya GG, Makarov VA, Zhidkov EA, Malykhina L.S., Sergeeva O.A., Ter-Arutyunyants A.A., Makarova L.V. Topical hemostatic agents (review). Pharmaceutical Chemistry Journal. 2006;40(7):9–15 (In Russ.). https://doi.org/10.30906/0023-1134-2006-40-7-9-15
13. Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. International Journal of Biological Macromolecules. 2017;105:1358–68. https://doi.org/10.1016/j.ijbiomac.2017.07.087
14. Dowling MB, Kumar R, Keibler MA, Hess JR, Bochicchio GV, Raghavan SR. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials. 2011;32(13):3351–7. https://doi.org/10.1016/j.biomaterials.2010.12.033
15. Okamoto Y, Yano R, Miyatake K, Tomohiro I, Shigemasa Y, Minami S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003;(53):337-42. https://doi.org/10.1016/s0142-9612(01)00324-6
16. Paneva D, Manolova N, Rashkov I, Danchev D, Gel beads composed of chitosan and polyacids and their blood compatibility. J. Bioact. Compat. Polym. 2005;(20):133–51. https://doi.org/10.1177/0883911505051855
17. Wang X, Yan Y, Zhang R A comparison of chitosan and collagen sponges as hemostatic dressings. J. Bioact. Compat. Polym. 2006;(21):39–54. https://doi.org/10.1177/0883911506060201
18. Samokhvalov IM, Golovko KP, Grishin MS, Nosov AM, Lyabakh DD, Kovalevskiy AYa. Experimental study of a local hemostatic agent based on chitosan and external abdominal compression for the temporary control of intra-abdominal bleeding. Journal of Emergency Surgery named after I.I. Janelidze. 2023;1:66–72 (In Russ.). https://doi.org/10.54866/27129632_2023_1_66
19. Mironov AN, ed. Guidelines for conducting preclinical studies of medicinal products. Moscow: Grif & K; 2012. (In Russ.).
20. ГОСТ ISO 10993-11-2021 Изделия медицинские. Оценка биологического действия медицинских изделий. Часть 11. Исследования общетоксического действия. ГОСТ ISO 10993-11-2021 Изделия медицинские. Оценка биологического действия медицинских изделий. Часть 11. Исследования общетоксического действия.
21. Men’shikov VV, Delektorskaya LN, Zolotnitskaya RP. Laboratory methods of research in the clinic. Moscow: Medicine; 1987 (In Russ.).
22. Lifshits VM, Sidel’nikova VI. Biochemical tests in the clinic: a handbook. Moscow: MIA; 1998 (In Russ.).
23. Rybakova AV, Makarova MN. Methods of euthanasia of laboratory animals, in accordance with European directive 2010/63. International Journal of Veterinary Medicine. 2015;2:96-107 (In Russ.). EDN: ULGMCX
24. Yunkerov VI. Mathematical and statistical processing of medical research data. St.Petersburg: VMedA; 2002 (In Russ.).
25. Formation of a replenishable register of health-associated reference intervals of biometric traits of laboratory animals. Research report (interim). Institution Golikov Research Clinical Center of Toxicology under the Federal Medical Biological Agency, supervised by Verveda AB, executed by: Bazhanova ED, Generalova KR, Kubarskaya LG, Belskaya AB, et al. SPb.; 2021. 180 с. № GR NIR AAAA-A20-120041690023-2. Dep. in CITIS 09.12.2021, № ICRBS 221121300357-3 (In Russ.).
26. Ivanova EA, Dzyuman AN, Dvornichenko MV. Local biocompatibility and biochemical profile of hepatic cytolysis in subcutaneous implantation of polylactide matrices. Bulletin of Siberian Medicine. 2022;21(4):63–71 (In Russ.). https://doi.org/10.20538/1682-0363-2022-4-63-71
About the Authors
A. M. NosovRussian Federation
Saint Petersburg
A. A. Bondarenko
Russian Federation
Saint Petersburg
G. G. Katretskaya
Russian Federation
Saint Petersburg
K. P. Golovko
Russian Federation
Saint Petersburg
A. V. Shultz
Russian Federation
Saint Petersburg
M. V. Volkova
Russian Federation
Saint Petersburg
E. A. Zolotoverkhaja
Russian Federation
Saint Petersburg
L. G. Kubarskaya
Russian Federation
Saint Petersburg
E. D. Bazhanova
Russian Federation
Saint Petersburg
O. N. Gaikova
Russian Federation
Saint Petersburg
Review
For citations:
Nosov A.M., Bondarenko A.A., Katretskaya G.G., Golovko K.P., Shultz A.V., Volkova M.V., Zolotoverkhaja E.A., Kubarskaya L.G., Bazhanova E.D., Gaikova O.N. General toxic effect of a chitosan-based hemostatic agent implanted in rats. Extreme Medicine. 2024;26(4):49-57. https://doi.org/10.47183/mes.2024-26-4-49-57

















