Scroll to:
Correlation of blood proteome parameters to the number of certain intestinal microflora bacteria in healthy women
https://doi.org/10.47183/mes.2024-26-4-123-131
Abstract
Introduction. Human intestinal microflora fulfils a wide range of important functions for the body. It provides non-specific anti-inflammatory defense through the production of bacteriocins, organic acids and substances with bacteriostatic properties. It also stimulates eukaryotic cells to synthesize mucin and substances with antimicrobial activity, thus suppressing the development of inflammatory reactions in intestinal epithelial cells. These bacteria obviously act synergistically with immunocompetent intestinal cells undergoing changes in zero gravity conditions modeled using dry immersion. Regulatory and metabolic changes which occur during model experiments are reflected, inter alia, in the protein composition of the blood.
Objective. Identification of the relationship between the blood protein level and the amount of E. coli, Lactobacillus spp., Enterococcus spp. and Bifidobacterium spp. in the intestine using an experimental model of 3-day dry immersion for potential use as clinical recommendations for the correction of intestinal microflora, based on data from the proteomic profile of the blood.
Materials and methods. The study was conducted among six women aged 25–40 years. During 3-day dry immersion, the subjects were completely immersed in an immersion bath containing water at room temperature. Direct contact between the subjects’ skin and the water was excluded. During the study, fecal samples and capillary blood samples were taken from each of the participants. In order to assess the protein levels, chromatography-mass spectrometric analysis of samples of dried blood spots was performed using nano-HPLC Dionex Ultimate3000 combined with a timsTOF Pro mass spectrometer. The study of the number of intestinal bacteria was carried out using culture seeding of pre-diluted fecal samples on selective media according to a standard technique, followed by consideration of colonies.
Results. The regression model showed a relationship between the levels of individual proteins and representatives of the intestinal microflora. A statistically significant correlation was found between blood proteins ENO1 (r = 0.71), MYH9 and SPTA1 (r = –0.99) with the amount of E. coli; blood proteins EPB41, VCP, C8B, CCT2 (r = 0.74), FAH, YWHAE (r = –0.46) with the amount of Bifidobacterium spp. There was also a significant strong positive correlation between Lactobacillus spp. and proteins ENO1, CA2 (r =0.74) and S100A6 and HSPA4 (r =–0.87). The CALM2 protein (r = –0.76) correlated with the amount of Enterococcus spp.
For citations:
Komissarova D.V., Pastushkova L.Kh., Kashirina D.N., Ilyin V.K., Larina I.M. Correlation of blood proteome parameters to the number of certain intestinal microflora bacteria in healthy women. Extreme Medicine. 2024;26(4):123-131. https://doi.org/10.47183/mes.2024-26-4-123-131
INTRODUCTION
Normal human intestinal microflora is represented by a wide range of microorganisms, most of which are obligate or facultative anaerobes. Opportunistic infections can occur directly due to obligate pathogens or indirectly due to excessive growth of opportunistic microorganisms. Depletion of the intestinal commensal population may also play a determining role [1]. Factors of space flight, for example, a changed diet, hygienic procedures, psycho-emotional stress, constant microbial metabolism, which inevitably occurs in a hermetically sealed space of a spacecraft, negatively affect the composition of the intestinal microbiota. This process is associated with active reproduction of the conditionally pathogenic component of the microflora, decreasing the number of protective types of microorganisms [2]. This requires the development of means to prevent and reduce the risks of developing dysbiotic conditions, as well as research into better understanding the interrelationships of the intestinal microbiota and other physiological and biochemical indicators of human health. This understanding will subsequently enable the composition of the intestinal microbiota to be influenced through targeted effects on individual processes in the body, for example, on the metabolism of single proteins.
The vast majority of bacteria live in the large intestine. The proximal sections of the small intestine normally contain up to 10⁴ CFU/mL of microorganisms, associated with the milieu pH (7.2–7.6) and the bactericidal effect of bile. The commensal microflora, in addition to participating in the digestive processes, fulfils a whole range of important functions for the host body. It provides non-specific anti-inflammatory defense through the production of bacteriocins, organic acids and substances with bacteriostatic properties. It also stimulates eukaryotic cells to synthesize mucin and substances with antimicrobial activity. In addition, the community of commensal microflora specifically suppresses the development of inflammatory reactions in intestinal epithelial cells. These representatives of the intestinal microflora obviously act synergistically with the local immune system [3][4].
Consequently, the human intestine is not only an important part of the digestive system for digesting food, absorbing water and nutrients, it also plays an essential role in the organization of immune defense [5]. The epithelial cells of the intestinal mucosa are involved in immune regulation. In the intestinal mucosa’s own plate, there are T and B cells which protect the body from pathogens. In addition, the cells of the intestinal mucosa produce various cytokines, such as gamma interferon (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 2 (IL-2) and interleukin 6 (IL-6), which are important regulators of both physiological adaptive reactions and congenital immune reactions [6]. In particular, they can participate in inflammatory reactions and mediate differentiation, proliferation and activation of various immune cells [7]. Moreover, the intestinal microflora is necessary for the organization and implementation of various immune reactions [8]. Thus, the microflora of intestinal commensals activates both innate and adaptive immunity in the cells of the intestinal mucosa [9].
In this study, we have attempted to analyze the possible relationship of the blood protein complex with the number of intestinal bacteria in women participating in an experiment with 3-day dry immersion [10].
Previous studies of the human intestinal microflora in a dry immersion experiment established significant deterioration in the state of the microflora, an increase in the proportion of opportunistic microorganisms, and a decrease in the amount of intestinal commensal bacteria [11].
According to some researchers, the regulatory and metabolic changes which occur during dry immersion experiments are reflected in blood protein composition. Mass spectrometry-based studies were performed by means of proteomics methods. Changes were established in the levels of plasminogen, fibronectin, other coagulation and fibrinolysis factors, as well as an increase in the content of fibrinolysis products, and activation of the complement system [12]. Proteomic methods clearly allow identification of proteins which respond to a complex set of dry immersion factors and clarify the molecular mechanisms of changes in various physiological systems.
The aim of the study was to identify the relationship between human blood proteins level and the amount of E. coli, Lactobacillus spp., Enterococcus spp. and Bifidobacterium spp. in the intestine. The study was conducted by means of experimental 3-day dry immersion. Its potential use is in clinical recommendations for the correction of intestinal microflora, based on data from the proteomic profile of the blood.
MATERIALS AND METHODS
Experimental design
Six women aged from 25 to 40 years participated in the 3-day dry immersion experiment. During the experiment, the subjects did not take antibacterial drugs or other drugs which can affect fluctuations in microflora levels. At the beginning of the experiment, all participants were assigned to the same phase of the menstrual cycle (follicular phase) in order to avoid differences in estradiol levels and its effects on microflora and plasma proteins. During the period of dry immersion, the subjects were not subjected to any additional influences, in the aim of preventing adaptive changes in physiological systems [10].
The dry immersion experiment is a method of simulating such factors affecting the body in space flight as hypogravity, support unloading, and redistribution of body fluids in the cranial direction. During dry immersion, the female subjects were in the immersion bath completely immersed in water at room temperature. Waterproof film prevented the skin of the subjects from coming into contact with water, permitting the time spent in the bath to be increased (Fig. 1).
The study was conducted on the Dry Immersion bench base of the Institute of Biomedical Problems. Throughout the experiment, the participants remained in a horizontal position without physical exertion and with limited voluntary movements.
The photo is published with the written consent of the participants of the study
Fig. 1. Volunteer in an immersion bath
Collection of stool samples, cultivation and identification of representatives of the intestinal microflora
Stool samples were taken 1–2 days before the start of the experiment and 1–3 days after the end of the dry immersion, in order to assess the amount of E. coli, Bifidobacterium spp., Lactobacillus spp., Enterococcus faecium. A number of tenfold dilutions were prepared from fecal samples in sterile saline solution from 10-1 to 10-9. Then 100 µL of the inoculate was sown in Petri dishes with selective nutrient media: De Man–Rogosa–Sharpe agar (MRS for cultivation of bacteria of the genus Lactobacillus spp.); Endo medium (for the cultivation of E. coli); agar for enterococci; and bifidoagar (manufacturer of all media — Himedia, India). The cultures were grown in a thermostat at 37°C for 48–72 h. Depending on the culture under study, bifidobacteria and lactobacilli were grown under anaerobic conditions. The colonies thus grown were counted using the Stegler SCM-2 colony counter and visually identified [13].
Collecting samples of dry blood stains
Capillary blood samples with a volume of 20 µL for chromatography-mass spectrometric analysis were taken using an automatic scarifier by piercing the terminal phalanx of the ring finger. This was followed by application to special filters (Perkin Elmer) for drying (the “dry spot” method). Blood samples were collected from volunteers 2 days before the start of the experiment and in dynamics for 1st, 2nd, 3rd days during the dry immersion, as well as 2 days after completion. After collection, the capillary blood samples were dried at room temperature for 2 h. Then the dried blood stain samples were stored at minus 20°C.
The dried blood stains were prepared for chromatography-mass spectrometric analysis, in order to determine proteins in human blood as follows: the proteins were extracted in a buffer containing 25 mmol ammonium bicarbonate, 1% sodium deoxycholate and 5 mmol TCEP (tris-(2-carboxyethyl) phosphine hydrochloride) (Thermo Scientific) at a temperature of 60°C, at a shaking rate of 1000 revolutions per minute (thermomixer, Eppendorf) for 1 h. They were then reduced, alkylated, precipitated and cleaved with trypsin, as described in the procedure [14].
Chromatography-mass spectrometric analysis of extracts of dry blood stains
Mixtures of tryptic peptides were separated using liquid chromatography based on nano-HPLC Dionex Ultimate3000 (Thermo Fisher Scientific, USA). They were then analyzed on a timsTOF Pro mass spectrometer (Bruker Daltonics, USA) using the method of parallel accumulation with sequential fragmentation (PASEF) [15].
Statistical analysis
The statistical analysis of the data obtained was carried out using a number of nonparametric techniques. Changes in the intestinal microflora were assessed using the nonparametric Kruskal-Wallis test for related samples. The eubiotic index was calculated by summation of positive and negative quantitative changes in protective and conditionally pathogenic groups of microorganisms. The index reflects positive changes in the composition of the microflora. Statistical processing of the eubiotic index was carried out using a paired two-sample t-test for averages.
The change in the amount of blood proteins was assessed using discriminant analysis for small samples. The relationship between the level of human blood proteins and the number of intestinal bacteria was suitably described using a regression model. In this model the number of bacteria was the dependent variable, while the number of proteins was the independent variable [16]. The results were processed using the Statistica 12.0 software package. P ≤ 0.05 was taken as the critical significance level. The STRING database was used to visualize protein relationships.
RESULTS
Approximately 1256 proteins were identified in the samples of dry blood spots of the female volunteers. Their relative levels were determined using the label-free quantification method. The regression model showed a relationship between the number of proteins in the blood described below and the number of bacteria E. coli, Bifidobacterium spp., Lactobacillus spp., Enterococcus faecium.
As a result of the regression analysis, the relationship of a number of proteins to the amount of E. coli was established (Fig. 2). The amount of ENO1 protein (alpha-enolase) in the blood positively correlated with the amount of E. coli (r = 0.71), while for the proteins MYH9 (non-muscular myosin with heavy chain IIa), ACLY (ATP citrate lyase), DPP3 (dipeptidyl peptidase 3), SPTA1 (spectrin alpha chain) a strong negative correlation r = –0.99 (p ≤ 0.05) was detected.
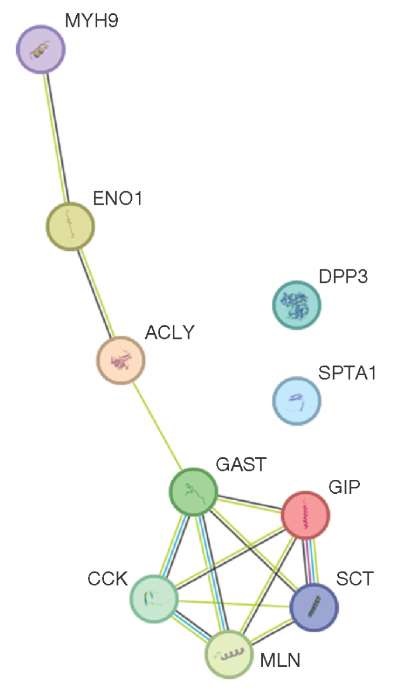
This figure was prepared by the authors using own data
Fig. 2. Relationship between blood proteins and number of E. coli in the intestinal microflora of the volunteers
The proteins EPB41 (Erythrocyte Membrane Protein Band 4.1), A1BG (Alpha-1-B Glycoprotein), VCP (Valosin Containing Protein), C8B (complement component C8 beta chain), and CCT2 (T-complex protein 1 subunit beta) were statistically significantly correlated with the number of bifidobacteria (Bifidobacterium spp.) in the intestine: r = 0.74 (p ≤ 0.05). Furthermore, a weak negative correlation r = –0.46 (p ≤ 0.05) was observed for proteins FAH (enzyme Fumarylacetoacetate hydrolase) and YWHAE (Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Epsilon). The relevant data is shown in Figure 3.
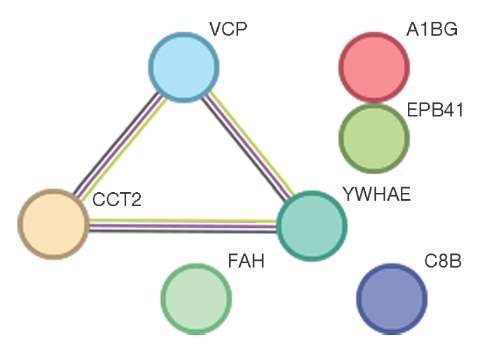
This figure was prepared by the authors using own data
Fig. 3. Interconnection of proteins which correlate to the number of Bifidobacterium spp. in the intestinal microflora of the volunteers
During the study, the data analysis established a significant strong positive correlation between the number of lactobacilli and blood proteins ENO1, CA2 (r = 0.74), and a negative correlation between the number of Lactobacillus spp. and proteins S100A6 and HSPA4 (r = –0.87). A negative correlation was also found between the CALM 2 protein (calmodulin) and the number of enterococci in the intestinal flora (r = –0.76) (p = 0.05). Table 1 summarized the data regarding the established correlation of proteins with some representatives of the intestinal microflora.
Table 1. Correlations of proteins with some representatives of the intestinal microflora revealed in the study, (p ≤ 0.05)
|
№ |
A microorganism of the intestinal microbiome |
Proteins negatively correlating with this microorganism |
r |
Positively correlating proteins with this microorganism, correlation coefficient |
r |
|
1 |
E. coli |
MYH9, ACLY, DPP3, SPTA1 |
0.99 |
ENO1 |
0,71 |
|
2 |
Lactobacillus spp. |
S100A6, HSPA4 |
0.87 |
ENO1, CA2 |
0,97 |
|
3 |
Enterococcus spp. |
CALM2 |
0.76 |
- |
|
|
4 |
Bifidobacterium spp. |
YWHAE, FAH |
0.46 |
VCP, C8B, CCT2, EPB41, A1BG |
0,74 |
This table was prepared by the authors based on their own data
DISCUSSION
As a result of regression analysis, the relationship of a number of proteins with the amount of E. coli was established. The amount of ENO1 protein (alpha-enolase, glycolytic enzyme that catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate) positively correlates to the amount of E. coli. The main functions of this protein are participation in glycolysis, cell growth processes, and allergic reactions. In addition, this protein serves as a receptor on the surface of leukocytes, thus stimulating the production of immunoglobulins [17].
It must be noted that E. coli plays an important role in the human body. It is capable of producing a number of vitamins (B1, B2, B6, K, etc.) and fatty acids. It participates in the metabolism of cholesterol, bilirubin, choline, bile acids and is involved in the absorption of iron and calcium [3][5].
E. coli has been shown to produce a substance with immunological similarity to somatostatin [16]. Somatostatin is also produced by D-cells of the small intestine, while somatostatin-28 is involved in the inhibition of insulin, secretin, glucagon, gastrin, and other hormones in the gastrointestinal tract. The main function of somatostatin synthesized in the intestine is to prevent the secretion of hydrochloric acid, slow intestinal motility, and change the level of bile acids [18].
The secretion of the large intestine contains a significant number of rejected epithelial cells, lymphocytes and mucus, although containing a small amount of enzymes. The Lieberkühn gland (crypt), along with intestinal villi, is one of the two most important structural units of the intestinal mucosa. For each villus in humans, there are from 4 to 7 Lieberkühn glands. The maximum number is located in the duodenum. The Lieberkühn glands of the large intestine are lined with a single-layered cylindrical polar epithelium, the height of which is higher at the mouth than at the base. The epithelium of the Lieberkühn glands contains various endocrine cells: I-cells producing cholecystokinin (CCK), S-cells secretin (SCT), K-cells glucose-dependent insulinotropic polypeptide (GIP), M–cells motilin (MLN), and G-cells gastrin (GAST) [19]. The above proteins are biochemically related to one another and to the ACLY protein (ATP-citrate lyase), to the level of which E. coli correlates. In addition, ACLY and a number of differentially expressed genes are involved in ErbB (erythoblastic oncogene B) signaling and cholicytokinin/gastrin signaling. ACLY is an important enzyme which binds carbohydrates to lipid metabolism by producing acetyl-CoA from citrate for the biosynthesis of fatty acids and cholesterol [20]. Changes in ACLY levels are probably related to gastrin signaling.
Another protein which affects the amount of E. coli is MYH9 (non-muscular myosin with heavy chain IIa, a member of the family of motor proteins). MYH9 is involved in the processes of secretion, cytokinesis and ensures cell mobility. The amount of E. coli negatively correlates with the amount of this blood protein, which may also be related to gastrin signaling.
After analyzing the data obtained, it can be concluded that an increase in the level of ENO1 and a decrease in MYH9 and ACLY contribute to an increase in the number of E. coli. The ENO1, MYH9 and ACLY genes are co-expressed and involved in gastrin signaling. Gastrin, in turn, is associated with cholecystokinin, secretin, glucose-dependent insulinotropic polypeptide and motilin, produced by the Lieberkühn glands of the colon. At the same time, glucose-dependent insulinotropic polypeptin (incretin) inhibits the absorption of fats, probably causing an increase in their amount in undigested food. As a result, this can lead to an increase in the amount of E. coli, for which fatty acids are one of the energy sources [21]. Incretin also inhibits lipoprotein lipase. According to Scholl RA et al., a high amount of E. coli correlates with a decrease in this enzyme [22].
Positively correlated to the amount of E. coli, the ENO1 protein stimulates the production of immunoglobulins, possibly indicating an increase in immune activity locally in the large intestine. One explanation for this relationship is the assumption that the autologous commensal intestinal microflora probably possesses tolerance to locally secreted immunoglobulins. A similar relationship was noted for another obligate representative of the intestinal flora: Lactobacillus spp. Summarizing the above, ENO1, MYH9 and ACLY are closely related to the processes of excretion of biologically active substances by cells of the Lieberkühn glands of the colon, in particular incretin (GIP). This, in turn, taking into account its functions, contributes to an increase in the number of E. coli.
The protein DPP3 (Dipeptidyl peptidase 3) is a zinc-dependent peptidase and an intracellular serine peptidase. This protein has a site of unique catalytic sequence which ensures the degradation of oligopeptides with residues from 4 to 10 amino acids. In our study, this site had a fairly strong negative correlation to the amount of E. coli. The DPP3 protein is known to have a wide range of biological functions. Thus, it participates in the intracellular cleavage of proteins. In addition, in some studies [23] have shown activity of DPP3 in cells of the innate immune system, for example, in polymorphonuclear granulocytes and neutrophils. This activity partly confirms its active participation in the regulation of the immune function of the body. It is interesting to note that our study established a strong negative correlation between the amount of this protein and both the amount of E. coli and the number of hemolytic staphylococci.
The study also found that the level of SPTA1 protein had a fairly strong negative correlation to the amount of E. coli. However, given the functions of this protein, the possible causes of the interaction of the levels of this protein and the number of E. coli representatives remain unclear.
Bifidobacteria are one of the most important components of the intestinal microflora. They are involved in the synthesis of lactate and acetate which regulate the pH of intestinal contents. They also provide increased colonization resistance of the intestinal microflora.
In our study, the strongest associations with the number of bifidobacteria in the intestine were found for the proteins EPB41, A1BG, VCP, C8B, and CCT2. A weak correlation was also established with the number of proteins FAH and YWHAE.
The protein encoded by the EPB41 gene (Erythrocyte Membrane Protein band 4.1) is a multifunctional protein which mediates interactions between the cytoskeleton of erythrocytes and the plasma membrane. The protein encoded by the EPB41 gene binds and stabilizes dopamine receptors D2 and D3 on the plasma membrane of neurons. It also participates in the regulation of calcium ion transport and regulation of intestinal absorption [24]. In the studies conducted, a positive correlation was established between the amount of EPB41 protein and the number of bifidobacteria. It was also noted that with a decrease in the amount of EPB41 protein, there is a violation of calcium absorption in the cells of the small intestine [23]. Thus, with an increase in the amount of EPER41 protein, calcium absorption in the epithelium of the small intestine increases. A similar process is controlled by bifidobacteria which also cause increased absorption of calcium ions. The largest amount of Bifidobacterium spp. is found in the large intestine. Bifidobacteria and lactobacilli make up about 20–30% of the microflora of the small intestine, localized mainly in the jejunum [25].
One of the putative functions of the A1BG protein (Alpha-1-B Glycoprotein) expressed in the liver is to participate in cell recognition and regulation of cellular behavior [26]. For this protein, a positive correlation was noted with the number of bifidobacteria. This correlation can be explained by tolerance of the immune system towards intestinal commensal population and an increased immune response against the background of a stress factor (dry immersion). It is important to note that the C8B protein (complement component C8 beta chain, lectin activation pathway), which also plays a key role in the implementation of the mechanisms of innate and adaptive immune response, negatively correlates with the number of bifidobacteria in the intestine.
CCT2 protein (T-complex protein 1 subunit beta, molecular chaperone) promotes protein folding during ATP hydrolysis. As part of the TRiC (chaperonin) complex, it plays a role in the folding of actin and tubulin. According to literature sources, the spectrin cytoskeleton is a target for intestinal bacterial pathogens (for example, pathogenic strains of E. coli, S. Typhimurium, L. Monocytogene) due to increased cell adhesion. It also plays a crucial role in the progression of dysbiotic conditions [27]. A similar mechanism may also enhance adhesion of Bifidobacterium spp. on the intestinal epithelium and, thus, promote their growth. In our study, a positive correlation was noted between the amount of this protein and the number of bifidobacteria.
At the same time, YWHAE (Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Epsilon), involved in the regulation of a wide range of both general and specialized signaling pathways, is also involved in the implementation of various biochemical processes related to signal transmission, such as cell division and regulation of insulin sensitivity. Its level is closely related to the amount of HSF1 protein (Heat shock factor 1). Its production by the cell, in turn, is induced not only by temperature stress, but also by many other provoking factors, namely hypoxic conditions, exposure to xenobiotics, proteotoxic stress [28]. The relationship between the levels of YWHAE blood protein and the amount of Bifidobacterium spp. in the intestinal flora is negative. Thus, with increased exposure to stress factors and an increase, respectively, in the YWHAE blood protein, the growth of bifidobacteria is inhibited. This inhibited growth confirms the relationship between stress levels of various etiologies and intestinal flora.
The FAH protein (enzyme Fumarylacetoacetate hydrolase which degrades 4-fumarylacetoacetate to acetoacetate and fumarate, providing the final stage of tyrosine amino acid catabolism), pursuant to its main function, is involved in tyrosine metabolism and is the last of the five enzymes which degrade this amino acid. The effect of this hydrolase and the conversion of 4-fumarylacetoacetate into fumarate and acetoacetate is to increase the blood level of ketone bodies. It has been shown that an excess of ketone bodies, for example, in keto diet, reduces the number of bifidobacteria. Thus, according to Ang QY, there is a negative correlation between them [29]. Our experiment showed a direct positive correlation between the amount of FAH protein and the amount of Bifidobacterium spp., although its severity was relatively weak.
At the same time, the VCP protein (valosin-containing protein) ATPase of the transitional endoplasmic reticulum is a component of the protein degradation process associated with the endoplasmic network. It is also responsible for maintaining cell proteostasis, including intestinal endothelium. Some studies have suggested that the VCP protein may contribute to the course of infection caused by toxoplasma [30]. This protein positively correlates to the number of bifidobacteria. In addition, it is co-expressed with CCT2 and YWHAE proteins.
Lactobacilli play an important role in maintaining the colonization resistance of the gastrointestinal tract. Although most bacteria in the gastrointestinal tract live in the large intestine, lactobacilli and enterococci are the dominant flora in the duodenum and jejunum. They are small in number compared to the colon (about 10³–10⁴ CFU/mL). However, they play an important role in immunomodulation and exhibit antagonistic activity against pathogenic microorganisms [31].
Most Lactobacillus spp. strains inhabiting the human intestine are homofermentative and form mainly lactic acid as a result of fermentation. They use glycolysis to form lactate from glucose. In relation to oxygen, most lactobacilli are aerotolerant anaerobes, that is, they grow most actively with oxygen deficiency.
In our study, the data analysis established a relationship between the number of proteins ENO1, CA2, S100A6, and HSPA4 and the number of lactobacilli. At the same time, ENO1 and CA2 had a positive correlation, and S100A6 and HSPA4 had a negative correlation to the amount of Lactobacillus spp.
Some studies have found that ENO1 is a protein which provides cell tolerance to hypoxia, while catalyzing the conversion of 2-phosphoglycerate to phosphoenolpyruvate during glycolysis. It is also present both in human cells and tissues, and in some species of Lactobacillus spp. The relationship between the amount of this protein and the number of lactobacilli is most likely primarily due to an increase in the tolerance of intestinal cells to hypoxia, which, in turn, contributes to an increase in the number of lactobacilli.
The protein CA2, belonging to the family of carbonic anhydrases, plays a crucial role in the functioning of hemoglobin. This is due to the catalysis of the process of hydration of carbon dioxide with the formation of carbonic acid and its subsequent dissociation in water. This leads to a decrease in the pH of the blood, which, in turn, reduces the affinity of hemoglobin to oxygen. Thus, an increase in the amount of this protein is also associated with hypoxia and, as a result, the creation of the most favorable conditions for the reproduction of Lactobacillus spp.
A6 S100A6 (S100) calcium-binding protein plays an important role in calcium binding. The level of this blood protein negatively correlates with the number of lactobacillus (Lactobacillus spp.), which use calcium ions in the process of citrate metabolism [32]. Consequently, with an increase in the amount of S100A6 protein, the amount of calcium necessary for the metabolism of lactobacilli decreases and the level of Lactobacillus spp. decreases.
It has been established that HSPA4 protein (a member of the Hsp110 family of heat shock proteins) possesses the important functions of a molecular chaperone inside the cell: response to an unfolded protein, protein import into the outer membrane of mitochondria, and assembly of protein complexes. Under the influence of many stress factors which cause the disruption of proteostasis processes, a decrease in intestinal commensals also occurs [33][34]. Our studies established that the correlation of the amount of this protein to the amount of Lactobacillus spp. was negative: the greater the level of this blood protein, the higher the stress level and the lower the number of lactobacilli.
Enterococci are one of the important components of the intestinal microflora. A number of drugs currently used as probiotics contain Enterococcus faecium. The number of enterococci in the intestine should normally be 10⁶ CFU/mL. Enterococci (along with lactobacilli), in addition to the large intestine, colonize the small intestine, albeit in a noticeably lesser number. Enterococcus spp. is classified as lactic acid microorganisms, since they perform fermentation-type metabolism and ferment carbohydrates to form lactic acid, which, in turn, reduces the milieu pH. In addition, enterococci are well-known producers of antimicrobial peptides (enterocins).
The analysis of the data obtained established a negative correlation between the protein CALM2 (calmodulin) in the blood and the number of enterococci in the intestinal flora. Calmodulin is expressed by epithelial cells in almost all parts of the small intestine and probably regulates the concentration of free calcium in microvilli cells. There is evidence that an increased content of calcium and magnesium ions in the medium inhibits enterocin production and enterococcal metabolism [35]. This is probably one of the possible reasons for the negative correlation of the CALM2 blood protein and the amount of Enterococcus spp. in the intestinal flora.
CONCLUSIONS
- The studies identified protein complexes the level of which in the host’s blood correlated to the number of protective microorganisms.
- All proteins correlating with different protective microorganisms can be conditionally divided into four groups depending on the functions and nature of the interaction of these proteins with various microorganisms: proteins associated with the immune system; proteins which directly or indirectly affect the processes of digestion and mineral metabolism; proteins which affect the tolerance of cells to hypoxia; proteins with a high regression coefficient in correlation with some microorganisms, but no obvious functional relationship.
- The data obtained contributes not only to a fundamental understanding of the relationship between the various processes in the human body, but can also serve as an important starting point for the formation of clinical recommendations for the correction of intestinal microflora based on data from the proteomic profile of blood, or, conversely, correction of blood protein parameters using probiotic and autoprobiotic agents.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows: Daria V. Komissarova — statistical processing of results, writing the section results, materials and methods, discussion of results, conclusions, Ludmila Kh. Pastushkova — design of the experimental part on proteomics, writing the section introduction, results and discussion of results, Daria N. Kashirina — conducting the experimental part on proteomics, writing the section introduction, materials and methods, discussion of results, Vyacheslav K. Ilyin — design and conducting the experimental part on microbiology, Irina M. Larina — design of the experimental part on proteomics, writing the section results, discussion of results and conclusions.
References
1. Ilyin VK, Volozhin AI, Vikha GV. Colonization resistance of an organism in altered living conditions. Moscow: Science; 2005 (In Russ.).
2. Turroni S, Magnani M, Kc P, Lesnik P, Vidal H, Heer M. Gut microbiome and space travelers’ health: state of the art and possible pro/prebiotic strategies for long-term space missions. Front Physiol. 2020;11:553929. https://doi.org/10.3389/fphys.2020.553929
3. Reinoso Webb C, Koboziev I, Furr KL, Grisham MB. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology. 2016;23(2):67–80. https://doi.org/10.1016/j.pathophys.2016.02.002
4. Iacob S, Iacob DG, Luminos LM. Intestinal microbiota as a host defense mechanism to infectious threats. Front. Microbiol. 2019;9:3328. https://doi.org/10.3389/fmicb.2018.03328
5. Leser TD, Mølbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ. Microbiol. 2009;11(9):2194–206. https://doi.org/10.1111/j.1462-2920.2009.01941.x
6. Onyiah JC, Colgan SP. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016;73(22):4203- 4212. https://doi.org/10.1007/s00018-016-2289-8
7. Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018;19(6):1813. https://doi.org/10.3390/ijms19061813
8. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. https://doi.org/10.1016/j.cell.2014.03.011
9. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. https://doi.org/10.1126/science.1223490
10. Tomilovskaya E, Amirova L, Nosikova I, Rukavishnikov I, Chernogorov R, Lebedeva S, et al. The first female dry immersion (NAIAD-2020): design and specifics of a 3-day study. Front. Physiol. 2021;12:661959. https://doi.org/10.3389/fphys.2021.661959
11. Ilyin VK, Komissarova DV, Morozova YuA, Zhiganshina AA. Changes in the intestinal microflora, upper respiratory tract and vaginal mucous membranes in volunteers in an experiment with «3-day «dry» immersion. Conference proceedings of the 56th scientific readings dedicated to the development of the scientific heritage and the development of the ideas of K.E. Tsiolkovsky. Kaluga; 2021 (In Russ.).
12. Pastushkova LCh, Goncharova AG, Kashirina DN, Goncharov IN, Rukavishnikov IV, Brzhozovskiy AG, et al. Characteristics of blood proteome changes in hemorrhagic syndrome after headup tilt test during 21-day dry immersion. Acta Astronautica. 2021;189:158–65. https://doi.org/10.1016/j.actaastro.2021.08.044
13. Menshikov VV, ed. Clinical laboratory analytics. Moscow; 2013 (In Russ.).
14. Kashirinа D, Brzhozovskiy A, Sun W, Pastushkova L, Popova O, Rusanov V, et al. Proteomic characterization of dry blood spots of healthy women during simulation the microgravity effects using dry immersion. Front. Physiol. 2022;12:75329. https://doi.org/10.3389/fphys.2021.753291
15. Meier F, Brunner AD, Koch S, Koch H, Lubeck M, Krause M, et al. Online Parallel Accumulation–Serial Fragmentation (PASEF) with a novel trapped ion mobility mass spectrometer. Mol. Cell. Proteomics. 2018;17(12):2534–45. https://doi.org/10.1074/mcp.TIR118.000900
16. Kulaichev AP. Methods and tools of complex statistical data analysis: a tutorial. 5th ed. Moscow: INFRA-M; 2017 (In Russ.)
17. Jensen EA, Young JA, Mathes SC, List EO, Carroll RK, Kuhn J, et al. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: A new frontier for microbial endocrinology. Growth Horm. IGF Res. 2020;53–4:101333. https://doi.org/10.1016/j.ghir.2020.101333
18. Schubert ML. Gastric acid secretion. Curr. Opin. Gastroenterol. 2016;32(6):452–60. https://doi.org/10.1097/MOG.0000000000000308
19. Functional gastroenterology. Available from: https://www.gastroscan.ru/handbook/117/309
20. Feng X, Zhang L, Xu S, Shen AZ. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020;77:101006. https://doi.org/10.1016/j.plipres.2019.101006
21. Tazi A, Araujo JR, Mulet C, Arena ET, Nigro G, Pédron T, Sansonetti PJ. Disentangling host-microbiota regulation of lipid secretion by enterocytes: insights from commensals Lactobacillus paracasei and Escherichia coli. mBio. 2018;9(5):e01493–18. https://doi.org/10.1128/mBio.01493-18
22. Scholl RA, Lang C.H., Bagby G.J. Hypertriglyceridemia and its relation to tissue lipoprotein lipase activity in endotoxemic, Escherichia coli bacteremic, and polymicrobial septic rats. J. Surg. Res. 1984; 37(5): 394–401. https://doi.org/10.1016/0022-4804(84)90205-1
23. Grdisa M, Vitale L. Types and localization of aminopeptidases in different human blood cells. Int. J. Biochem. 1991;23(3):339–45. https://doi.org/10.1016/0020-711x(91)90116-5
24. Liu C, Weng H, Chen L, Yang S, Wang H, Debnath G, et al. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium ATPase 1b (PMCA1b). J. Biol. Chem. 2013;288(16):11407–15. https://doi.org/10.1074/jbc.M112.436659
25. Innovative food technologies. Available from: https://propionix.ru/kolichestvo-mikroorganizmov-v-tonkom-i-tolstom-kishechnike
26. Sun D, Zhao YY, Dai SP, Fang K, Dong LY, Ding Q. Cloning and analysis of human alpha-1B glycoprotein precursor gene: a novel member of human immunoglobulin superfamily. Acta Genetica Sinica. 2002;29(4):299–302. PMID: 11985261.
27. Ruetz T, Cornick S, Guttman JA. The spectrin cytoskeleton is crucial for adherent and invasive bacterial pathogenesis. PLOS ONE. 2011;6(5):e19940. https://doi.org/10.1371/journal.pone.0019940
28. Dayalan Naidu S, Dinkova-Kostova AT. Regulation of the mammalian heat shock factor 1. FEBS J. 2017;284(11):1606–27. https://doi.org/10.1111/febs.13999
29. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263–75.e16. https://doi.org/10.1016/j.cell.2020.04.027
30. Clough B, Fisch D, Mize TH, Encheva V, Snijders A, Frickel E-M. p97/VCP targets Toxoplasma gondii vacuoles for parasite restriction in interferon-stimulated human cells. bioRxiv. 2023.06.20.545566. https://doi.org/10.1101/2023.06.20.545566
31. Yarullina DR, Fakhrullin RF. Bacteria of the genus Lactobacillus: general characteristics and methods of working with them. Study guide of the Kazan (Volga Region) Federal University. Kazan; 2014 (In Russ.)
32. Mortera P, Pudlik A, Magni C, Alarcón S, Lolkema JS. Ca2+- citrate uptake and metabolism in Lactobacillus casei ATCC 334. Appl. Environ. Microbiol. 2013;79(15):4603–12. https://doi.org/10.1128/AEM.00925-13
33. Lutgendorff F, Akkermans LM, Söderholm JD. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr. Mol. Med. 2008;8(4):282–98. https://doi.org/10.2174/156652408784533779
34. Mardanov AV, Babykin MM, Beletsky AV, Grigoriev AI, Zinchenko VV, Kadnikov VV, et al. Metagenomic analysis of the dynamics of changes in the composition of the intestinal microbiome of participants in the Mars-500 experiment simulating a long-term space flight. Acta Naturae. 2013;3(48):120–8.
35. Kumar M, Srivastava S. Effect of calcium and magnesium on the antimicrobial action of enterocin LR/6 produced by Enterococcus faecium LR/6. Int. J. Antimicrob. Agents. 2011;37(6):572–5. https://doi.org/10.1016/j.ijantimicag.2011.01.014
About the Authors
D. V. KomissarovaRussian Federation
Moscow
L. Kh. Pastushkova
Russian Federation
Moscow
D. N. Kashirina
Russian Federation
Moscow
V. K. Ilyin
Russian Federation
Moscow
I. M. Larina
Russian Federation
Moscow
Supplementary files
Review
For citations:
Komissarova D.V., Pastushkova L.Kh., Kashirina D.N., Ilyin V.K., Larina I.M. Correlation of blood proteome parameters to the number of certain intestinal microflora bacteria in healthy women. Extreme Medicine. 2024;26(4):123-131. https://doi.org/10.47183/mes.2024-26-4-123-131

















