Scroll to:
Prospects for the use of intranasal nanoscale polymer delivery systems for drugs and antidotes in extreme medicine
https://doi.org/10.47183/mes.2024-26-4-27-37
Abstract
Introduction. The development of improved formulations of antidotes and remedies, which can be used not only by qualified medical personnel, but also in self- and mutual assistance, is an urgent task for extreme medicine.
Objective. Evaluation of the possibility of using nanoscale polymer delivery systems for medicines and antidotes intended for intranasal administration (into the nasal cavity) in extreme medicine.
Discussion. The main submicron-sized polymer carriers which are promising as the basis for the creation of an intranasal form of antidotes are identified. The bioavailability of the substance delivered is dependent on the physico-chemical properties of the carrier, the conditions for its production, as well as physiological and anatomical factors. Data is presented regarding possible ways of correcting these factors in order to increase bioavailability. Examples of the use of polymer nanocarriers in the treatment of poisoning with heavy metals and rocket fuel components, as well as lesions caused by radioactive substances, are presented. It is shown that carriers (dendrimers, cyclodextrins) can act as antidotes in certain cases. The study presents a list of antidotes approved for use within the territory of the Russian Federation, for which the development of intranasal forms is possible, taking their physico-chemical and pharmacokinetic properties into account.
Conclusions. Following a review of literature sources, the most promising submicron-sized polymer carriers for the intensification of intranasal delivery of drugs and antidotes are herein proposed: dendrimers, liposomes, nanocapsules, nanoparticles, and cyclodextrins. Using the list of antidotes approved for use in the Russian Federation as an example, promising drugs that can be potentially developed on the basis of these carriers are proposed.
For citations:
Fedotova E.V., Krivorotov D.V., Radilov A.S. Prospects for the use of intranasal nanoscale polymer delivery systems for drugs and antidotes in extreme medicine. Extreme Medicine. 2024;26(4):27-37. https://doi.org/10.47183/mes.2024-26-4-27-37
INTRODUCTION
In pharmacology/toxicology, an antidote is usually any drug which can neutralize a toxicant and eliminate its pathological effect. For speed of action, such drugs are usually developed and produced in formulations of injection solutions. Despite the successes and achievements of the modern pharmaceutical industry, for objective pharmacoeconomical reasons, there is now more than ever a shortage in both the global pharmaceutical market and in Russia of a number of medicines used in the treatment of acute and chronic poisoning with heavy metals, narcotic and organophosphorus compounds (OPCs), cyanides and hydrazine.
Antidote therapy is part of the comprehensive treatment of acute poisoning of both a domestic and technogenic nature. It is used by specialists in toxicological teams at the pre-hospital stage, physicians of medical institutions at the hospital stage, as well as in the order of self- and mutual assistance in peacetime and wartime.
Over the past decade [1], the growing need for convenient and effective medicines for the treatment and relief of intoxication effects has led to significant progress in pharmaceutical technologies. The number of improved formulations of antidotes and remedies has increased significantly. They can be used not only by qualified medical personnel, but also in self- and mutual assistance. A growing trend is the use of modern delivery systems to modify the detoxification properties of antidotes. When developing new formulations of known antidotes, differences need to be taking into account regarding the pharmacokinetic parameters relative to traditional, mainly injectable drugs. The greatest interest in this aspect is linked to the use of intranasal forms of medicines. They allow the neutralization of the differences in speed between invasive and non-invasive dosage forms of administration, albeit not proving adequate bioavailability and therapeutic effect in all cases. One of the possible solutions for the development of intranasal medicines is the use of polymer micro- and nanocapsules as carriers for antidote delivery. This formulation is one of the current trends in the treatment of metal poisoning, as well as the prevention of severe poisoning with narcotic substances and alcohol [2][3].
The development of non-invasive dosage forms of antidotes, in particular intranasal forms using polymer nanocarriers, may not only increase the effectiveness of known drugs, but also expand the scope of their medical use. However, the drug delivery system, including nanoscale delivery, should not change the initial properties of the antidote/drug substance or lead to side effects [4]. For example, this could be respiratory depression caused by the undesirable delivery of loperamide to the central nervous system (CNS) resulting from co-administration with P-glycoprotein inhibitors [5]. On the contrary, the most promising application of the intranasal form of drug administration in the treatment of acute poisoning with neurotoxicants is due to its potential to intensify the transport of the active substance into brain tissue, bypassing the blood-brain barrier (BBB) and the metabolism phase in internal organs. For example, in the case of poisoning with OPCs, the low ability of pralidoxime to penetrate through the BBB as an antidote has little effect on centrally mediated respiratory depression caused by the OPC action. However, when administered intranasally in the form of cationic liposomes, pralidoxime chloride (2-pyridine aldoxime methyl chloride or 2-PAM) as a carrier is able to reduce brain damage and mortality in rats with their poisoning with paraoxone [6].
Intranasal forms of antidotes may be promising in the choice of therapy for poisoning caused by pulmonotoxicants, vesicants and nerve agents, as well as in acute radiation injuries [7][8]. Therefore, for the relief of nausea and vomiting syndrome, the nasal form of antiemetics significantly simplifies the provision of assistance to the victim, when the administration of traditional remedies in the form of dispersible tablets or buccal films would be impossible.
Modern nanocarriers proposed for drug modification include: nanoparticles such as carbon nanoparticles (nanotubes, graphenes); non-carbon nanoparticles (iron, gold particles); nanoparticles made of biopolymers (capsules, liposomes), nanorobots and nanochips [9–12]; dendrimers — three-dimensional branched monodisperse polymers; and clathrates — beta cyclodextrin complexes. Liposomes are biocompatible and biodegradable bilayer lipid vesicles up to 0.5 microns in size, capable of encapsulating both polar and nonpolar compounds [13][14].
The main distinguishing characteristic of these nanomaterials is their size and composition. They initially determine the primary physical and chemical properties of the future carrier: solubility in water and biological fluids, surface charge, sorption, aggregation and adhesion abilities, intermolecular interactions, interaction with cell membranes and proteins, cytotoxicity [15].
Drugs based on polymer micro- and nanocarriers can be administered in various ways: orally, buccally, transdermally, nasally, parenterally, etc.
In the case of intranasal administration, the absorption of substances occurs mainly in the nasal cavity [16][17] and depends on the various factors shown in Figure 1, the features of which and ways to correct them will be discussed further.
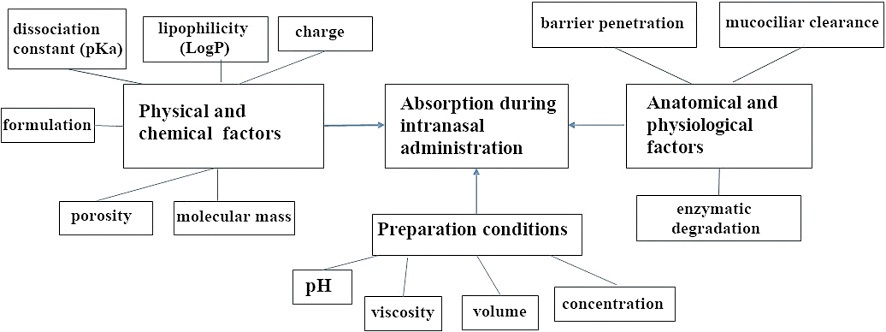
The figure was prepared by the authors based on own data [15]
Figure 1. Factors affecting the absorption of drug delivery systems during intranasal drug administration
A characteristic feature of intranasal administration is the absence of the effect of the first passage through the liver. Due to the anatomical features of the nasal mucosa, substances enter directly into the arterial bloodstream. High bioavailability, ease of use and high rate of effect development allow this route of administration to be considered as promising for the delivery of drugs based on polymer micro- and nanocarriers.
This work is aimed at evaluating the possibility of using nanoscale polymer delivery systems for medicines and antidotes intended for injection into the nasal cavity (intranasally) in extreme medicine.
RESULTS AND DISCUSSION
Physical and chemical factors of substance absorption in the intranasal administration route and methods of their correction
The bioavailability of substances in the intranasal administration route depends of the physical and chemical properties of nanocarriers, such as surface-to-mass ratio, strength, conductivity, solubility, stability, and reactivity [17].
The development of synthetic carriers capable of capturing target biomacromolecules is one of the directions for creating a new generation of antidotes. In nature, interactions between biomacromolecules are realized due to weak electrostatic, hydrophobic, hydrogen, and Van der Waals forces. When creating antidote carriers capable of capturing target molecules, multipoint electrostatic interactions are simulated by including functional monomers (chelating ligands). For example, nanoparticles made of poly(ethylene-co-glycidylmethacrylate) functionalized with triethylenetetramine, N,N-di(2-pyridylmethyl)amine, 8-hydroxyquinoline or 8-hydroxyquinoline-2-sulfonic acid can be used as antidotes for copper poisoning [18].
Polymer capsules can include hydrophilic and hydrophobic antidotes, forming both covalent and non-covalent bonds with them. Polymer nanocapsules are able to protect the antidote from its degradation caused by blood proteins or enzymes (peptidases, phospholipases) of the blood-brain barrier. The release of the antidote from micro- and nanocapsules depends on the physico-chemical characteristics of the drug (particle size, concentration and solubility) and the polymer itself (structure, molecular weight, porosity and mechanical strength).
Anatomical and physiological factors of substance absorption in the intranasal administration route and methods for their correction
The physical chemical characteristics have a significant impact on the further pathways of the drug after intranasal administration. If the drug is more than 1 kDa in size, does not dissolve in the nasal mucosa, or has a pronounced negative charge (repels from a negatively charged mucous membrane), then it will not be able to penetrate the mucous membrane of the nasal sinuses. The penetration of substances through the mucous membrane can occur in several ways, for example, transcellular penetration and paracellular (between cells). Lipophilic substances carry out transcytosis by passive diffusion and are rapidly absorbed by vesicular transport mechanisms. Therefore, the use of lipophilic delivery systems for non-lipophilic antidotes can significantly increase their effectiveness. Polar antidote delivery systems pass through the epithelium paracellularly. The latter is less effective for large molecules (more than 1000 Da) [16].
The possibility of axonal transport, which enables bypassing of the BBB, is important for the intranasal delivery of many analgesics and antispasmodics, for example, as well as for the relief of various syndromes caused by the action of toxicants. However, with this pathway, the carrier must be capable of retrograde and anterograde movement in axons and dendrites [19]. Important physical chemical characteristics of nanocarriers in this penetration pathway are their size and chemical composition of the surface. Nanoparticles of 20–50 nm in size are able to penetrate directly into the central nervous system by axonal transport and can enhance bioavailability [20]. Negatively charged nanocarriers are attracted by the membranes and synaptic slits of neurons. Therefore, such carriers may be applicable for the intraneural delivery of drugs. Positively and neutrally charged nanocarriers are characterized by slow axonal transfer, while negatively charged carriers are characterized by rapid transfer [21].
The effect of the production conditions of dosage forms on the substance absorption in the intranasal administration route
Substance absorption during the intranasal administration route depends on the production conditions of a nanoscale delivery system. The development of each separately selected system also requires an individual approach and a study of its toxicity. The use of toxic organic solvents (as in the case of the use of ethylene glycol in the formation of some nanocapsules) the desired size reduction to be achieved. However, in certain cases, it can lead to increased toxicity if not completely removed. The method for obtaining a nanocarrier is based on the physical and chemical properties of the drug included. The selected method of preparation should not negatively affect the substance carried (contribute to its degradation or modify its properties).
A competent choice of the nanocarrier polymer material includes the following conditions: polymers should not be toxic, carcinogenic or mutagenic, and should be biocompatible. Some branched polymers (polypropylenimine dendrimers (PPI), polyamidamine (PAMAM) and polylysine (PLL)) are known to have significant cytotoxicity due to the high content of terminal amino groups [22]. Therefore, the most often used carriers are no higher than the 4th generation. In most cases, the toxic effects caused by polymer nanocarriers are associated with an increase in their cytotoxicity, namely: a decrease in cell viability, an increase in apoptosis, DNA destruction, rupture of the cell membrane and activation of lipid peroxidation [23].
One of the significant disadvantages of intranasal administration of most drugs is the relatively low permeability of the nasal mucosa to large macromolecules and intensive mucociliary clearance. The use of bioadhesive polymers (such as chitosan, carbopol, cyclodextrin and pluronic), which are part of the nanocarrier, will increase the residence time of the drug in the nasal cavity, thereby improving absorption. The nature of the polymer affects its bioadhesive qualities. In addition, the bioadhesive polymer must be polar and have sufficient viscosity. Polymer mucoadhesion is significantly dependent on the flexibility of the polymer chain (rigid crosslinking of polymer chains significantly limits their diffusion through membranes and interaction with mucin), molecular weight (the lower the molecular weight, the easier the permeability through the mucosa), the degree of swelling, and the ability to form hydrogen bonds.
Since the nasal mucosa is lipophilic, the degree of absorption of lipophilic carriers can be assumed to be more effective. In order to ensure adequate pharmacokinetics of the polymer necessary for the realization of the therapeutic effect during intranasal administration, special solvents and penetrant substances which increase the ability to penetrate biological membranes are used, as well as various systems of carrier particles of various nature, shape, and size. For example, chitosan supplementation can increase the paracellular transport of substances with intranasal administration of 0.5% chitosan and 1% atropine sulfate drops in the treatment of organophosphorus poisoning [24]. Cyclodextrin (dimethyl-beta-cyclodextrin) may also be of interest as a carrier due to its ability not only to transfer medicinal substances, but also, like chitosan, to increase their paracellular transport [25].
Despite a number of significant differences in the pharmacokinetics of parenteral and intranasal methods of administration, the latter has a significant advantage, namely, noninvasiveness of the antidote administration procedure, the possibility of dose reduction due to better bioavailability, simplification of production technology, and the possibility of self-administration [18][20].
At the same time, promising carriers can simultaneously deliver various groups of drugs (for example, antiemetics and analgesics). The use of carriers for medicines enables their physical and chemical properties (increase hydrophilicity, permeability of biological barriers) to be modified, and bioavailability increased, thereby optimizing the pharmacokinetic parameters.
From the entire variety, we selected what we consider to be four of the most promising submicron carriers for the nasal administration route: liposomes, dendrimers, nanocapsules, and cyclodextrins.
Use of nanocarriers to intensify the penetration of drugs and antidotes through the blood-brain barrier
Many substances dangerous to humans (narcotic substances, organophosphorus pesticides, nerve agents, etc.) easily overcome the blood-brain barrier (BBB) and penetrate into the tissues of the central nervous system. At the same time, only a small part of the medicines used in medicine are capable of effectively overcoming the BBB, which makes it difficult for antidotes for CNS-active substances to be developed. In most cases, only hydrophilic compounds with a molecular weight of less than 150 Da and hydrophobic compounds with a mass of less than 600 Da are able to penetrate the BBB by passive diffusion [26–28]. Thus, many of the drugs used are not able to provide effective protection of the central nervous system in cases of intoxication of various origins.
The penetration rate of drugs through the BBB determines the adequacy, timeliness, and effectiveness of therapeutic action [28]. For example, the experimentally established rate of naloxone intake into the brain is 8–10 times higher than the rate of morphine intake [29], which explains its pronounced antidote effect. However, in the case of poisoning by centrally active toxicants rapidly penetrating through the BBB, the antidote activity of naloxone is significantly reduced [30]. At the same time, the combination of effective delivery systems and nasal administration can significantly enhance the effect of traditional antidotes and ensure their use not only by qualified medical personnel, but also in self- and mutual assistance [28].
Nanocarrier-based delivery systems can provide drugs with improved penetration efficiency through the BBB in four ways: by accumulating on the walls of brain blood capillaries, thus increasing the concentration gradient of the antidote between the bloodstream and central nervous system tissues; by passing in free form or together with the carrier due to the disclosure of dense connections between brain endothelial cells; by endocytosis of endothelial cells and the subsequent release of the antidote in the endothelial layer and diffusion into the brain tissue; and through transcytosis through a layer of endothelial cells into the brain [31]. Nanoparticles made of biodegradable polymers such as polylactide glycolide (PLGA), human serum albumin (HSA), and chitosan are most often used as carriers for drug delivery to the brain [32]. For example, quercetin, used as an antidote to arsenic, encapsulated in PLGA nanoparticles, is capable of crossing the BBB, which enables the depletion of brain cells in poisoning [33] to be neutralized.
Dendrimers (three-dimensional branched monodisperse polymers) capable of passing through the BBB have proved to be promising polymer nanocarriers for antidotes of neurotoxicants [32]. Thus, conjugates of the low molecular weight antidote of the OPC pyridine-aldoxime with polyamidamine dendrimer (PAMAM) of the 5th generation have shown their effectiveness in a mice model of paraxone intoxication [34]. Polyester dendrimers 2,2-bis(hydroxymethyl)propanoic acid (bis-MPA) are capable of binding organophosphorus compounds, in particular dichlorvos, allowing such a polymer nanocarrier to be considered as an independent antidote to OPC [35].
The use of polymer nanocarriers for heavy metals poisoning
The group of heavy metals includes mercury, lead, cadmium, arsenic, chromium, cobalt, molybdenum, nickel, antimony, zinc, scandium, manganese, vanadium, strontium, barium, and tungsten. Their organic and inorganic compounds are found in many industries and are actively used in agriculture and everyday life. The mechanism of action of many heavy metals is based on the blocking of sulfhydryl, amine, and carboxyl groups of proteins-enzymes and structural proteins. As a result, protein, carbohydrate, and fat metabolism in the body is disrupted. The development of new antidotes and chelates for the treatment of heavy metal intoxication remains an important and pressing task [36].
Chelation therapy is one of the ways to treat heavy metal poisoning. It is based on the formation of an insoluble, less toxic metal complex easily excreted from the body. Therefore, a promising direction in the treatment of heavy metal poisoning is the use of polymer nanoparticles both as delivery carriers and independently, taking the chelating properties of these compounds into account.
Cyclodextrins are natural polymer delivery systems. They are representatives of a separate class of macrocyclic ligands in supramolecular chemistry [36], characterized as non-toxic substances capable of forming complexes with many toxicants (Fig. 2).
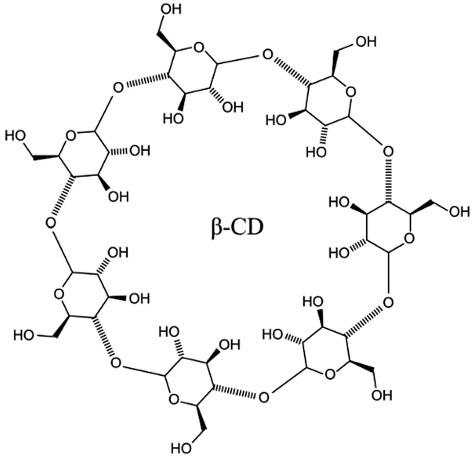
The figure was prepared using PubChem data [37]
Figure 2. Structural formula of beta-cyclodextrin
The use of cyclodextrin as an independent antidote became known for the first time in 2002, when its ability to neutralize the effects caused by muscle relaxants was demonstrated [38]. This allowed the development of a drug for the reversal of neuromuscular blockade based on modified gamma-cyclodextrin administered intravenously at doses from 1 to 16 mg/kg. The administration route and dosage allow the possibility of an intranasal route of cyclodextrin administration to be considered as an alternative, particularly given that these compounds have the ability to bind to many xenobiotics, in particular opioid analgesics. Therefore they can be used as detoxifying substances in poisoning with long-acting opiates such as methadone, for which there are no available antidotes [39]. Since the outer surface of natural cyclodextrins contains primary and secondary hydroxyl groups which can covalently bind to heavy metal ions, they can also act as promising antidotes for poisoning with heavy metals such as copper, lead (the strongest affinity) and cadmium [40]. Since certain cyclodextrins are also allowed for use in the food industry in the production of specialized food products, they can be recommended as a therapeutic and preventive nutrition for employees of enterprises in contact with heavy metals.
Chelation therapy with dimercaptosuccinic acid (DMSA), ethylenediaminetetraacetic acid (EDTA), 2,3-dimercaptopropanol (BAL), and D-penicillamine is proposed for the treatment of chronic poisoning with heavy metals (in particular lead). However, their main disadvantages include weak solubility in water, low bioavailability when ingested (orally), and a short half-life, which significantly limits their clinical use [41]. At the same time, heavy metal adsorbents used in industry, such as mesoporous silicon nanoparticles (MSN) [10], can potentially be used as a drug adsorbent in the body, for example, in the treatment of cases of poisoning with thiol poisons. Thus, MSN nanoparticles modified with EDTA chelator showed a good effect on iron intoxication [42]. Curcumin encapsulated in chitosan nanocapsules with a diameter of 50 nm is also being considered for the treatment of heavy metal poisoning. The chitosan shell protects the compound from absorption by the reticuloendothelial system (RES), increases its bioavailability, and allows longer circulation in the blood. This approach permits the dose of curcumin to be significantly reduced when administered orally for the effective removal of heavy metals from the body [43]. Similarly, encapsulation of selenomethionine in PLGA nanocapsules resulted in a 7-fold increase in its detoxifying efficacy against mercury-containing substances compared with the traditional method of administration [44].
The use of dendrimers and dendrigrafts as carriers enables certain hydrophobic substances to be rendered hydrophilic. Their use in complexation with water-soluble chelators such as DMSA allows hydrophilic complexes with optimal bioavailability [41] to be obtained. Water-soluble complexes of dendrimers with polyphenol quercetin provide the ability to penetrate the BBB, thus being promising for combating oxidative stress in arsenic poisoning [33][45].
Use of polymer nanocarriers for the delivery of antidotes and radioprotectors
Pyridoxine (vitamin B6) is considered the only effective antidote for poisoning with unsymmetrical dimethylhydrazine (UDMH) [46]. This drug relieves convulsive syndrome and reduces the toxic effect of UDMH and its metabolites on the central nervous system. The neurotoxic effect of asymmetric dimethylhydrazine is manifested in a decrease in the content of pyridoxal phosphate due to its interaction with pyridoxal contained in brain tissue cells. As a result, toxic pyridoxal hydrazones are formed which inhibit the activity of pyridoxal kinase and thereby block the synthesis of pyridoxal phosphate in the cell [46].
The structure of vitamin B6 contains a hydroxyl group, suggesting the possibility of electrostatic interaction with polymer carriers having positively charged functional groups, for example, dendrimers. The polyphenol curcumin, enclosed in a nanoliposomal form (NLC), has also proven itself as a therapy for dimethylhydrazine poisoning in mice. The administration of nanoliposomal curcumin at a dose of 150 mg/kg significantly reduced serum alanine aminotransferase (ALT) and lactate dehydrogenase (LDH). This increased under the action of dimethylhydrazine, at the same time as significantly increasing the level of gamma-aminobutyric acid (GABA) in the hippocampus [47].
Salts of hydrocyanic acid are highly dangerous to humans. At the same time, liposomal methemoglobin (MetHb@Lipo) has shown its effectiveness as a new antidote for cyanide poisoning [48], significantly increasing the survival of animals after contact with hydrogen sulfide. This is achieved by maintaining the activity of cytochrome C oxidase and suppressing metabolic acidosis [13].
Damage by radioactive substances can occur only in non-standard emergency situations (waste transportation, testing and work on machine with radioactive elements). The development of a non-invasive form of radioprotectors is also an important task. For example, Аmifostin, the most well-known and effective radioprotector, can currently be administered only parenterally, but with this method of administration it is quickly eliminated from the body. Therefore, researchers around the world are actively developing a new form of antidote which could provide more effective dosing of the drug and reduce its toxicity. Mandal TK et al. developed hybrid microcapsules of PLGA and chitosan, enabling Amifostine to be effectively capsulated. This composition of the microcapsule ensured a 45% reduction in the drug release rate. In addition, the introduction of chitosan into the shell is believed to increase the absorption of the drug and increase its bioavailability [49].
Use of polymer nanocarriers for the development of symptomatic therapy
A number of chemicals cause poisoning by a wide range of pathophysiological life-threatening processes. In such cases, the simultaneous use of both etiotropic therapy and pathogenetic, symptomatic therapy is required, in order to eliminate individual symptoms of intoxication. The improvement of therapeutic drugs for symptomatic therapy through the development of delivery systems can presumably not only significantly facilitate the procedure of their administration, but also increase the effectiveness of treatment. For example, modified antiemetic drugs can be used to inhibit the gag reflex in acute radiation injuries and OPC poisoning. Currently, the classic routes of administration of this group of drugs are oral and parenteral. The intranasal administration of antiemetics can significantly simplify the procedure of drug administration and neutralize the effect of the first passage through the liver [50]. In the study by Ozsoy Y, and Gungör S, nasal administration of metoclopramide in-situ in the formulation of poloxamer 405 gels increased its bioavailability by 19% compared with oral administration [51]. The high level of efficiency with intranasal administration of ondancetron and granisetrone was obtained due to their inclusion in chitosan microparticles crosslinked with glutaraldehyde [52]. Granisetron preparation encapsulated in microparticles based on cyclodextrin and carboxymethylcellulose showed high antiemetic activity during intranasal administration [53].
In the case of poisoning with irritating substances, burns, and in dental and surgical practice, analgesics (opioid and non-opioid analgesics of central action, adjuvant analgesics, nonsteroidal anti-inflammatory drugs) are used to treat pain. However, many analgesics have a short period of action, leading to an increase in the administration frequency to the patient. In this case, the use of a delivery system may allow for a gradual low-dose controlled intake of the drug into the victim’s body while maintaining effectiveness and reducing side effects. For example, the inclusion of Benzocaine® in polymer nanoparticles (PLGA, PLA, PCL) makes it possible to prolong the analgesic effect when compared with a conventional drug [54].
No less important in acute poisoning with many toxicants is the relief of convulsive syndrome. The use of anticonvulsants, such as carbamazepine in the formulation of carboxymethyl chitosan nanoparticles, with intranasal administration makes it possible to ensure the necessary concentration of the drug in the brain and, accordingly, increase the effectiveness of treatment [55].
Proposals for the modification of antidotes approved for use in the Russian Federation
Based on the review of domestic and foreign literature, as well as taking the structural and physical and chemical characteristics of drugs recommended for use as antidotes into account, we have proposed possible polymer carriers for modifying some antidotes currently allowed for use in the Russian Federation (Table 1).
Table 1. Assessment of the prospects for the development of non-invasive formulation of certain antidotes approved for use in the Russian Federation, taking into account their physical and chemical and pharmacokinetic properties
|
Antidote name and indications |
Formulation, dose |
Physical and chemical properties |
Absorption |
Feasibility of developing non-invasive dosage forms |
|
Galantamine
Cholinolytic poisoning |
Sol. for intravenous and subcutaneous administration 1 mg/mL |
M = 368.27 g/mol Slightly soluble in water and alcohol It is soluble in chloroform pKa 8.32 |
Galantamine relieves cerebral cholinergic syndrome. The absolute oral bioavailability is about 90%. Half-life: 7 h |
Liposomes and dendrimers with a particle size of 3–5 microns effectively deliver drugs to the brain. Based on the physical and chemical properties of the drug, the development of its liposomal formulation by hydration/rehydration of a thin film is promising due to its weak solubility in water and alcohol, but good solubility in chloroform. The reactive hydroxyl group of galantamine can bind electrostatically to dendrimers having positively charged amino groups, in particular to a third-generation polylysine dendrimer capable of penetrating the BBB. The resulting electrostatic bond is rather fragile and will allow for a smooth release of the drug from the carrier molecule. Effective delivery of the drug to the central nervous system is expected with intranasal administration, which may make it possible to provide the necessary therapeutic dose of galantamine. |
|
Glucagon® NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser- Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu- Met-Asn-Thr-COOH Overdose with calcium channel blockers |
Lyophilisate for injection. 1 mg |
M = 3485 g/mol Hydrophobic The solutions of the drug are stable for 48 h at a 5°C pKa 7,1 |
With i/v glucagon 1 mg/mL, the maximum blood level 7.9 ng/mL is reached after 20 min. With i/m administration, the maximum level 6.9 ng/mL is reached after 13 min. With intranasally glucagon 3 mg, the maximum level 6130 pg/mL is reached after 15 min. |
Consideration of the structure, physical and chemical properties of glucagon allows us to expect the inclusion of this peptide in the hydrophobic part of the liposome, which can provide its protection from the effects of enzymes and degradation. The charged reactional end groups of the peptide (NH2 and COOH) suggest the possibility of formation of electrostatic interactions with charged dendrimers. When administered intranasally, such liposome-based or dendrimer-based drugs can provide the required concentration of glucagon necessary to ensure therapeutic action. |
|
Carboxim®
OPC poisoning |
Solution for injection 15% — 1 mL (150 mg) |
M = 413.35 g/mol Hydrophilic |
Central nervous system cholinesterase reactivator. Restores neuromuscular conduction. |
Due to its structure, Carboxim does not pass through the BBB well. A large dose is required for intramuscular delivery of this drug. The use of a properly selected drug carrier can significantly reduce its high dose while maintaining the therapeutic effect. At the same time, ensuring optimal bioavailability with intranasal administration of Carboxim may make it possible to create an antidote convenient for use within the framework of self- and mutual assistance. Some functionalized cyclodextrins are stoichiometrically capable of absorbing OPC. The intranasal delivery system «acetylcholinesterase reactivator — cyclodextrin» will ensure the paracellular transport of the drug and, probably, will make it possible to increase its effectiveness. Since Carboxim and other acetylcholinesterase reactivators are hydrophilic drugs, they can be included in both nanocapsules and liposomes. However, the most promising solution is the formation of a dendrimer-carboxim complex due to charged fragments of the molecule. This can increase the efficiency of the passage of carboxim through the BBB. In addition, the dendrimer is able to react independently with some OPC, which will also increase the effectiveness of the antidote. |
|
Naloxone
Narcotic analgesics poisoning |
Injection for solution, 0.4 mg/mL, 1 mL, ampoules |
M = 327.4 g/mol Hydrophilic pKa 7.9 |
μ-opioid receptors antagonist Half-life: 1-1.5 h Oral bioavailability is up to 20% |
Liposomal and dendrimeric formulations of opioid receptor antagonists with intranasal administration make it possible to maximize the bioavailability of active substances by increasing their absorption by simple diffusion, necessary for a reliable therapeutic effect in cases of poisoning with narcotic analgesics. At the same time, the lack of bioavailability with this method of administration is compensated by the direct entry of antagonists into the tissues of the central nervous system which provides a therapeutic effect no worse than that of their injectable drugs. On the contrary, in the treatment of socially significant diseases (various types of addictions), microcapsulation in biodegradable polymers such as PLGA or chitosan is the most promising, which usually provides a slow prolonged effect of such drugs when administered in depot form and provides their protection from the effects of RES. |
|
Nalmefene
Alcohol addiction treatment |
Film-coated tablets, 18 mg |
M = 339.4 g/mol Hydrophilic pKa 7.6 |
A long-acting opioid antagonist with affinity for the k-opioid receptor and the m-opioid receptor. It is not subjected to presystemic metabolism. |
The table was prepared by the authors using PubChem data [37]
Obviously, there is no a need to develop all antidotes need in an intranasal form. For example, drugs such as ethyl alcohol or calcium gluconate were initially excluded from our analysis. Moreover, the development of intranasal forms for some antidotes is initially undesirable, since it can enhance the effect of the drug and lead to possible dangerous side effects. For example, in the case of the anticholinesterase drug proserin, it is undesirable to increase its effect on the central nervous system. Some of the antidotes used in medicine (such as atropine sulfate, Cuprenyl® and pyridoxine hydrochloride) with good bioavailability and efficacy in conventional forms do not need significant modifications, except for convenience of use. Nevertheless, the development of an intranasal form of pyridoxine, for example, may prove promising as a possible preventive antidote for workers who have professional contact with rocket fuel components. The structure of both pyridoxine and atropine technically allows them to bind electrostatically to the end groups of the dendrimer and, based on our assumptions, such a delivery system can demonstrate greater efficiency due to the intensification of absorption and penetration through the BBB. This will allow the antidote to be administered in emergency situations by means of self- and mutual assistance.
In some cases, a less invasive intranasal form may be an alternative to parenteral and oral administration and find its application, for example, in pediatrics. A well-known antidote such as Vikasol is used for overdose with vitamin K antagonist drugs (warfarin, fenindione, acenocumarol). The development of intranasal dosage forms of synthetic vitamin K may have potential in pediatrics for the prevention of vitamin K-dependent hemorrhagic syndrome in newborns. Parenteral administration of the antidote to children with this syndrome leads to excessive traumatization, while the liposomal intranasal formulation of vitamin K will not only resolve the problem of simplifying the administration of the drug to patients, but also ensure its effective delivery to the body.
The expediency of developing non-invasive dosage forms currently approved for use in the Russian Federation [56], taking into account their physical and chemical and pharmacokinetic properties, is considered in Table 1.
CONCLUSION
The conducted research allows us to conclude that the most promising nanoscale polymer delivery systems for drugs and antidotes intended for intranasal administration are dendrimers, liposomes, cyclodextrins, and nanocapsules.
The absorption of the drug and antidote delivery system during intranasal delivery depends on the physical and chemical characteristics of the carrier (surface-to-mass ratio, strength, conductivity, solubility, stability, and reactivity), the physiological and anatomical factors (ability to penetrate the BBB), as well as the conditions for obtaining carriers (presence or absence of impurities in the form of solvents and penetrant substances).
It was shown that some carriers can act as antidotes. Thus, dendrimers are capable of reacting with OPC (as in the case of the bis-MPA polyester dendrimer and dichlorvos), while cyclodextrins are antidotes for muscle relaxants.
At the moment, not only modified chelating substances (such as mesoporous silicon nanoparticles modified with EDTA), but also chitosan nanoparticles containing curcumin and selenomethionine encapsulated in PLGA nanocapsules have shown a high level of efficiency in heavy metal poisoning.
The intranasal method of administration may be convenient for radiation injuries, when oral administration of the antidote is difficult (due to vomiting). Increasing the effectiveness of known radioprotectors and reducing their toxicity while simultaneously administering them non-invasively is also a promising and still unresolved area in this field.
The possibility of using polymer nanocarriers in order to improve therapeutic drugs for symptomatic therapy (antiemetics, anti-inflammatory, anticonvulsants, painkillers and antihistamines) was evaluated. The possibility of prolonging the analgesic effect of the drug is demonstrated by the example of inclusion of benzocaine in PLGA nanoparticles.
This review provides suggestions for modifying the reserve of antidotes used in the Russian Federation. For many of them, it is shown that the intranasal form of administration is the most promising due to its non-invasiveness (parenteral administration causes additional traumatization of the affected tissues and can serve as an entrance gate for secondary infection), ease of use, as well as the rapid speed of drug delivery. This work also notes that microcapsulation allows, if necessary, programmable release of the drug to be achieved. Most nanocarriers have both undeniable advantages and limitations. In this regard, the issues of searching for the most effective intranasal carriers remain open today.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows: Elena V. Fedotova — significant contribution to the collection and analysis of data for the work; drafting the work; agreeing to be responsible for all aspects of the work, ensuring that issues related to the accuracy or integrity of any part of the work will be properly investigated and resolved. Denis V. Krivorotov — significant contribution to the collection and interpretation of data for the work; critical analysis of the work for important intellectual content; agreement to be responsible for all aspects of the work, ensuring that issues related to the accuracy or integrity of any part of the work will be properly investigated and resolved. Andrey S. Radilov — significant contribution to the interpretation of data for the work; significant contribution to the design of the work; reviewing the work critically for important intellectual content; final approval of the version to be published; agreement to be responsible for all aspects of the work, ensuring that issues related to the accuracy or integrity of any part of the work will be properly investigated and resolved.
References
1. Pitschmann V, Hon Z. Drugs as Chemical Weapons: Past and Perspectives. Toxics. 2023;11(1):52. https://doi.org/10.3390/toxics11010052
2. Manek E, Petroianu GA. Brain delivery of antidotes by polymeric nanoparticles. Journal of Applied Toxicology. 2021;41(1):20–32. https://doi.org/10.1002/jat.4029
3. Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology. 2022;239(7):2063–81. https://doi.org/10.1007/s00213-022-06125-5
4. Wunnapuk K. Toxicology and Safety of Nanoparticles in Drug Delivery System. Fundamentals of Drug Delivery. 2021:329–48. https://doi.org/10.1002/9781119769644.ch13
5. Kharasch ED, Hoffer C, Whittington D. The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. British journal of clinical pharmacology. 2004;57(5):600–10. https://doi.org/10.1111/j.1365-2125.2003.02053.x
6. Pashirova TN, Zueva IV, Petrov KA, Lukashenko SS, Nizameev IR, et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids and Surfaces B: Biointerfaces. 2018;171:358–67. https://doi.org/10.1016/j.colsurfb.2018.07.049
7. Ivanov IM, Ivchenko EV, Yudin MA, Vengerovich NG, Nikiforov AS, et al. Application aspects of medications for inhalation at the prehospital stage of medical evacuation. Bulletin of the Russian Military Medical Academy. 2021;23(4):247–55 (In Russ.). https://doi.org/10.17816/brmma58989
8. Ivnitskiy YuYu, Krasnov KA, Ivanov MB, Reinyuk VL, Golovko AI. Prospects of nasal spray in the arsenal of first aid in acute poisoning. Toxicological bulletin. 2020;2(161): 4–10 (In Russ.). https://doi.org/10.36946/0869-7922-2020-2-3-9
9. Osman NS, Sabri MA, Sapawe N. Synthesis of Mesoporous Silica Nanoparticles from Oil Palm Frond Based Silica to Enhanced Removal of Heavy Metal. Trans Tech Publications Ltd. 2022;1076: 221–27. https://doi.org/10.4028/p-29dk6k
10. Laffleur F., Bauer B. Progress in nasal drug delivery systems. International Journal of Pharmaceutics. 2021;607:120994. https://doi.org/10.1016/j.ijpharm.2021.120994
11. Jain KK. An overview of drug delivery systems. Drug Delivery Systems. 2020:1–54. https://doi.org/10.1007/978-1-4939-9798-5_1
12. Aneebuddin MK, Kumar P. Recent Trends in the Chemistry of Polymers used in Oral Drug Delivery Systems. Chemistry Research Journal. 2022;7(6):97–106. https://doi.org/10.1186/s40824-020-00190-7
13. Suzuki Y, Taguchi K, Kure T, Enoki Y, Otagiri M, et al. Liposomal methemoglobin as a potent antidote for hydrogen sulfide poisoning. Toxicology and Applied Pharmacology. 2022;450:116159. https://doi.org/10.1016/j.taap.2022.116159
14. Suzuki Y, Taguchi K, Kure T, Sakai H, Enoki Y, et al. Liposome-encapsulated methemoglobin as an antidote against cyanide poisoning. Journal of Controlled Release. 2021;337:59–70. https://doi.org/10.1016/j.jconrel.2021.07.015
15. Illum L. Nasal drug delivery: new developments and strategies. Drug discovery today. 2002;7(23):1184–9. https://doi.org/10.1016/S1359-6446(02)02529-1
16. Rawal SU, Patel BM, Patel MM. New drug delivery systems developed for brain targeting. Drugs. 2022;82(7):749–92. https://doi.org/10.1007/s40265-022-01717-z
17. Pawar B, Vasdev N, Gupta T, Mhatre M, More A, et al. Current Update on Transcellular Brain Drug Delivery. Pharmaceutics. 2022;14(12):2719. https://doi.org/10.3390/pharmaceutics14122719
18. Weisman A, Chou B, O’Brien J, Shea KJ. Polymer antidotes for toxin sequestration. Advanced drug delivery reviews. 2015;90:81–100. https://doi.org/10.1016/j.addr.2015.05.011
19. Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? Journal of nanoscience and nanotechnology. 2009;9(8):4996-5007. https://doi.org/10.1166/jnn.2009.GR02
20. Wang W, Hassan MM, Mao G. Colloidal Perspective on Targeted Drug Delivery to the Central Nervous System. Langmuir. 2023;39(9):3235–45. https://doi.org/10.1021/acs.langmuir.2c02949
21. Romashchenko AV, Petrovskii DV, Trotsky SY, et al. Quantitative tracking of trans-synaptic nose-to-brain transport of nanoparticles and its modulation by odor, aging, and Parkinson’s disease. Nano Res. 2023;16:7119–33. https://doi.org/10.1007/s12274-022-5302-6
22. Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. Journal of Pharmacy and Bioallied Sciences. 2014;6(3):139–50. https://doi.org/10.4103/0975-7406.130965
23. Sairam AB, Sanmugam A, Pushparaj A, Mahesh Kumar G. Toxicity of polymeric nanodrugs as drug carriers. ACS Chemical Health & Safety. 2023;30(5):236–50. https://doi.org/10.1021/acs.chas.3c00008
24. Rajpal S, Mittal G, Sachdeva R, Chhillar M, Ali R, et al. Development of atropine sulphate nasal drops and its pharmacokinetic and safety evaluation in healthy human volunteers. Environmental Toxicology and Pharmacology. 2009;27(2):206–11. https://doi.org/10.1016/j.etap.2008.10.007
25. Haimhoffer Á, Rusznyák Á, Réti-Nagy K, Vasvári G, Váradi J, et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Scientia Pharmaceutica. 2019; 7(4):33. https://doi.org/10.3390/scipharm87040033
26. Praphawatvet T, Peters JI, Williams RO. Inhaled nanoparticles– An updated review. International Journal of Pharmaceutics. 2020;587:119671. https://doi.org/10.1016/j.ijpharm.2020.119671
27. Veronesi MC, Alhamami M, Miedema SB, Yun Y, Ruiz-Cardozo M, et al. Imaging of intranasal drug delivery to the brain. American journal of nuclear medicine and molecular imaging. 2020;10(1):1–31. PMCID: PMC7076302
28. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–53. https://doi.org/10.1602/neurorx.2.4.541
29. Uiba VV, Krivorotov DV, Zabelin MV, Radilov AS, Rembovsky VR. Opioid receptor antagonists. From the present to the future. Emergency medicine. 2018;20(3):356–370
30. Fishman J, Hahn EF, Norton BI. Comparative in vivo distribution of opiate agonists and antagonists by means of double isotope techniques. Life Sciences. 1975;17(7):1119–25. https://doi.org/10.1016/0024-3205(75)90333-1
31. Skolnick P. Treatment of overdose in the synthetic opioid era. Pharmacology & therapeutics. 2022;233:108019. https://doi.org/10.1016/j.pharmthera.2021.108019
32. Türker S, Onur E, Ózer Y. Nasal route and drug delivery systems. Pharmacy world and Science. 2004;26:137–42. https://doi.org/10.1023/B:PHAR.0000026823.82950.ff
33. Ghosh A, Mandal AK, Sarkar S, Panda S, Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life sciences. 2009;84(3-4):75–80. https://doi.org/10.1016/j.lfs.2008.11.001
34. Bharathi S, Wong PT, Desai A, Lykhytska O, Choe V, et al. Design and mechanistic investigation of oxime-conjugated PAMAM dendrimers as the catalytic scavenger of reactive organophosphate. Journal of Materials Chemistry B. 2014;2(8):1068–78. https://doi.org/10.1039/C3TB21267J
35. Duran-Lara EF, Marple JL, Giesen JA, Fang Y, Jordan JH, ́ et al. Investigation of lysine-functionalized dendrimers as dichlorvos detoxification agents. Biomacromolecules. 2015;16(11):3434–44. https://doi.org/10.1021/acs.biomac.5b00657
36. Yin H, Zhang X, Wei J, Lu S, Bardelang D, et al. Recent advances in supramolecular antidotes. Theranostics. 2021;11(3):1513. https://doi.org/10.7150/thno.53459
37. [Электронныйресурс]. URL: https://pubchem.ncbi.nlm.nih.gov/ (дата обращения: 26.04.2024)
38. Puskás I, Szente L, Szőcs L, Fenyvesi É. Recent List of Cyclodextrin-Containing Drug Products. Periodica Polytechnica Chemical Engineering. 2023;67(1):11–7. https://doi.org/10.3311/PPch.21222
39. Mayer BP, Kennedy DJ, Lau EY, Valdez CA. Evaluation of polyanionic cyclodextrins as high affinity binding scaffolds for fentanyl. Scientific Reports. 2023;13(1):2680. https://doi.org/10.1038/s41598-023-29662-1
40. He J, Li Y, Wang C, Zhang K, Lin D, et.al. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers. Applied Surface Science. 2017;426:29–39. https://doi.org/10.1016/j.apsusc.2017.07.103
41. Wang H, Yao Q, Zhu W, Yang Y, Gao C, et al. Biomimetic antidote nanoparticles: a novel strategy for chronic heavy metal poisoning. Aaps Pharmscitech. 2022;24(1):12. https://doi.org/10.1208/s12249-022-02466-8
42. Farjadian F, et al. In vitro and in vivo assessment of EDTA-modified silica nano-spheres with supreme capacity of iron capture as a novel antidote agent. Nanomedicine: Nanotechnology, Biology and Medicine. 2017; 13(2): 745–53. http://dx.doi.org/10.1016/j.nano.2016.10.012
43. Yadav N, Mudgal D, Anand R, Jindal S, Mishra V, et al. Recent development in nanoencapsulation and delivery of natural bioactives through chitosan scaffolds for various biological applications. International Journal of Biological Macromolecules. 2022;1(220):537–72. https://doi.org/10.1016/j.ijbiomac.2022.08.098
44. Hu X, Tulsieram KL, Zhou Q, Mu L, Wen J. Polymeric nanoparticle–aptamer bioconjugates can diminish the toxicity of mercury in vivo. Toxicology Letters. 2012;208(1): 69–74. https://doi.org/10.1016/j.toxlet.2011.10.006
45. Yousefi M, Narmani A, Jafari SM. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Advances in colloid and interface science. 2020;278: 102125. https://doi.org/10.1016/j.cis.2020.102125
46. Antushevich AE, Basharin VA, Reinyuk VL, Bugaev PA. Effectiveness of inosine glycyl-cysteinyl-glutamate disodium and pyridoxine hydrochloride in acute poisoning with asymmetric dimethylhydrazine. Bulletin of the Russian Military Medical Academy. 2018;1:164–67 https://doi.org/10.17816/brmma12289
47. Kulabhusan PK., Agrawa lS, Jeevanandam J, Danquah MK. Nanoformulated Herbal Drug Delivery as Efficient Antidotes Against Systemic Poisons. Poisonous Plants and Phytochemicals in Drug Discovery. 2020;269–94. https://doi.org/10.1002/9781119650034.ch13
48. Fan N, Li Q, Liu Y, Ma B, Li M, et al. Preparation of an HI-6- loaded brain-targeted liposomes based on the nasal delivery route and the evaluation of its reactivation of central toxic acetylcholinesterase. European Journal of Pharmaceutical Sciences. 2023;184:106406. https://doi.org/10.1016/j.ejps.2023.106406
49. Pamujula S, Graves RA, Moiseyev R, Bostanian LA, Kishore V, Mandal TK. Preparation of polylactide-co-glycolide and chitosan hybrid microcapsules of amifostine using coaxial ultrasonic atomizer with solvent evaporation. Journal of Pharmacy and Pharmacology. 2008; 60(3):283–89. https://doi.org/10.1211/jpp.60.3.0002
50. Chavda VP, Jogi G, Shah N, Athalye MN, Bamaniya N, et al. Advanced particulate carrier-mediated technologies for nasal drug delivery. Journal of Drug Delivery Science and Technology. 2022;74:19. https://doi.org/10.1016/j.jddst.2022.103569
51. Ozsoy Y, Güngör S. Nasal route: an alternative approach for antiemetic drug delivery. Expert Opinion on Drug Delivery. 2011;8(11):1439–53. https://doi.org/10.1517/17425247.2011.607437
52. Pandey J, Shankar R, Kumar M, Shukla K, Kumari B. Development of nasal mucoadhesive microspheres of granisetron: A potential drug. Drug Research. 2020;70(8):367. https://doi.org/10.1055/a-1193-4781
53. Ruby JJ, Pandey VP. Antiemetic drugs as a nasal drug deliveryA review. International Journal of Pharmaceutical Sciences and Research. 2014;(5):1624. https://doi.org/10.13040/IJPSR.0975-8232.5(5).1624-29
54. Wang B, Wang S, Zhang Q, Deng Y, Li X. Recent advances in polymer-based drug delivery systems for local anesthetics. Acta biomaterialia. 2019;96:55–67. https://doi.org/10.1016/j.actbio.2019.05.044
55. Liu S, Yang S, Ho PC. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian journal of pharmaceutical sciences. 2018;13(1):72–81. https://doi.org/10.1016/j.ajps.2017.09.001
56. Gladkikh VD, et al. Regulatory, legal, scientific and industrial aspects of the state and prospects of development of the antidote therapy system in the Russian Federation. Bulletin of the RCB Protection troops. 2023;2(4):10–21 https://doi.org/10.35825/2587-5728-2018-2-4-10-27
About the Authors
E. V. FedotovaRussian Federation
Leningrad region; St. Petersburg
D. V. Krivorotov
Russian Federation
Leningrad region
A. S. Radilov
Russian Federation
Leningrad region
Supplementary files
Review
For citations:
Fedotova E.V., Krivorotov D.V., Radilov A.S. Prospects for the use of intranasal nanoscale polymer delivery systems for drugs and antidotes in extreme medicine. Extreme Medicine. 2024;26(4):27-37. https://doi.org/10.47183/mes.2024-26-4-27-37