Scroll to:
Experimental model of convulsive syndrome based on phenylcarbamate
https://doi.org/10.47183/mes.2024-26-4-38-48
Abstract
Introduction. Carbamates are widely used in the pharmaceutical industry, agriculture, and household chemicals. Being reversible cholinesterase inhibitors, carbamates can cause the development of generalized convulsive syndrome. Untimely treatment contributes to the emergence of persistent neurological disorders. In order to develop and adequately assess in preclinical studies the specific activity of new drugs for the relief of convulsive syndrome in acute intoxication with this group of substances, an easily reproducible experimental model of convulsive carbamate-induced syndrome is required.
Objective. Development of an experimental model of generalized convulsive syndrome in rats using phenylcarbamate as a model toxicant for testing in preclinical studies of therapies for poisoning with cholinesterase inhibitors.
Materials and methods. The study was performed using mongrel sexually mature male rats, aged 3 months (80 animals), divided into 4 groups (3 experimental and 1 control). At the first stage, the parameters of convulsive syndrome caused by model toxicants were compared: phenylcarbamate 1 mg/kg bw, corazol 65 mg/kg bw, and thiosemicarbazide 8 mg/kg bw. The following parameters were studied: motor activity (open field test), neuromotor functions (grip strength test), cognitive functions (conditioned avoidance responses, CAR), and cardiovascular indicators (ECG and cardiac rhythmogram assessment). The severity of the convulsive syndrome was identified by Racine stages. Additionally, the structure of brain tissues was evaluated by histological methods. second stage, biochemical parameters were studied in three experimental (with toxicants) and control groups. Some biochemical parameters were studied in the blood serum, assessing the function of the liver, kidneys, prooxidant and antioxidant systems. At the third stage, the activity of cholinesterase in the blood and brain was studied in 30 control and 30 experimental rats after phenylcarbamate exposure. Statistical processing of the results was carried out using Statistica v.10.
Results. When modeling convulsive syndrome in rats, phenylcarbamate is comparable to corazol in terms of the onset of the latency period, duration and intensity of seizures. When implementing the model, a significant decrease in heart rate was recorded 48 h after administration. The CAR test found that the introduced substance increases the time of the first entry into the dark compartment before training. Significant changes in markers of liver function (ALT, bilirubin, cholesterol, triglycerides), lipid peroxidation and the antioxidant system (MDA, GPx) confirm the complexity of mechanisms responsible for the development of seizures and neurological disorders. The results of histological examination of brain tissues indicate that phenylcarbamate induces pronounced disorders of the brain structure in an experiment on rats.
Conclusions. The developed experimental model of phenylcarbamate-based convulsive syndrome in rats is easy to reproduce, thus being recommended for preclinical studies of new drugs for the relief of convulsive syndrome in poisoning with cholinesterase inhibitors.
Keywords
For citations:
Melekhova A.S., Belskaya A.V., Zorina V.N., Melnikova M.G., Kubarskaya L.G., Gaikova O.N. Experimental model of convulsive syndrome based on phenylcarbamate. Extreme Medicine. 2024;26(4):38-48. https://doi.org/10.47183/mes.2024-26-4-38-48
INTRODUCTION
Carbamates are derivatives of carbamic acid, the amino and carboxyl endings of which are replaced by structurally diverse alkyl, aryl, or alkyl-aryl groups [1]. Carbamates are structurally similar to amides and esters. Due to their chemical and conformational stability, resistance to proteolysis, as well as the ability of many representatives of the group to pass through the cell membrane and the blood-brain barrier, carbamates are increasingly used in the pharmaceutical industry both as an active component of dosage forms and as carriers replacing peptides in finished dosage forms [2]. In addition to the pharmaceutical industry, carbamates are actively used in agriculture and as part of household chemicals, which often causes accidental or even intentional poisoning.
A number of carbamic acid derivatives belong to highly toxic compounds, reversible (unlike organophosphorus compounds, OPs) cholinesterase inhibitors, leading to the formation of the so-called “cholinergic crisis” associated with the development of generalized convulsive syndrome, in severe cases ending in coma and death. Under the action of carbamates during acute intoxication, due to the rapid hydrolysis of the C = O bond (decarbomylation of the enzyme), the activity of cholinesterases in survivors is restored within several hours; complete restoration of cholinesterase function is observed after 24–48 h [3]. At the same time, poisoning with cholinesterase inhibitors results in inflammatory reactions in the tissues of the nervous system, apoptosis of nerve cells, and neurodegenerative changes that potentiate the development of persistent neurological disorders in survivors.
It should be noted that the levels of mortality and disability in acute carbamate poisoning are rather high. Thus, according to WHO data from 2019, more than 1600 people died globally as a result of deliberate suicide using carbamates alone, the number of non-fatal poisonings ranged from several hundred thousand to several million per year (according to statistics in different countries). As a result of ineffective or untimely treatment, a significant proportion of survivors subsequently experience tremors, dizziness, headaches, partial memory loss, emotional lability, confusion, cognitive impairment, peripheral neuropathy, and autonomic dysfunction [4].
It should be noted that the treatment of acute carbamate poisoning relies on the methods similar to those used in OP poisoning (atropinization, administration of benzodiazepine-type drugs to relieve convulsive syndrome in the initial 10–20 min after exposure) and methods of treatment of acute attacks of true epilepsy with the development of seizures refractory to benzodiazepines (administration of barbiturates, anesthetics) [5]. The effectiveness of antidotes is high only provided they are used at the earliest stages of poisoning [6].
Recently, an increasing amount of evidence indicates that the pathogenesis of carbamate and OP poisoning includes, among other things, manifestations of noncholinergic toxicity through the induction of reactive oxygen species (ROS) and the formation of carbonylated proteins. OPs and carbamates also interact with mitochondrial translocation proteins, with androgen, estrogen, and glucocorticoid receptors involved in cholesterol metabolism, negatively affecting their functions. At the same time, the mechanisms and severity of OP and carbamate effects on the body differ [7][8]. This justifies the need to develop improved therapies for carbamate poisoning. However, this task is impossible to achieve without adequate experimental models capable of assessing the specific activity of new drugs in preclinical studies. To date, the creation of anticonvulsants has been carried out mainly for the treatment of true epilepsy; maximum electric shock, corazol administration, etc., are used as experimental models [9]. Organophosphorus compounds (diazinon, malaoxon, chlorfenvinphos, and dichlorvos) are commonly used as toxicants–pesticides affecting cholinesterase activity in experiments to simulate generalized convulsive syndrome in animals [10]. Experimental models of generalized convulsive syndrome using carbamates, which impose significantly lower safety requirements during preclinical studies, have not been so far described in scientific publications.
Thus, in the present work, we set out to develop an experimental model of generalized convulsive syndrome in rats using phenylcarbamate as a model toxicant for testing of therapies for poisoning with cholinesterase inhibitors in preclinical studies.
MATERIALS AND METHODS
The study was conducted on white mongrel 3-month-old male rats, weighing 150–250 g, provided by PLZH “Rappolovo” of the National Research Center “Kurchatov Institute,” Leningrad Oblast, Russia. The animals were kept in standard vivarium conditions [11]. A 12 h lighting cycle was maintained; food and water were provided ad libitum.
In a series of preliminary experiments, the median lethal dose (LD50) of phenylcarbamate (PC) was determined for intraperitoneal and intragastric routes of administration to rats, amounting to 1.43 ± 0.12 mg/kg of body weight (bw) and 10.0 ± 0.77 mg/kg bw, respectively [12]. For higher reproducibility of the experimental model of convulsive syndrome in preclinical studies, the intraperitoneal route of administration of phenylcarbamate was selected. This route is an alternative to the intravenous route of administration, enabling 100% bioavailability of the drug. In our previous work, a convulsive dose of phenylcarbamate of 1 mg/kg bw was also experimentally selected, which ensures the induction of convulsive syndrome in 100% of animals with a minimum percentage of mortality.
The experiment consisted of three consecutive stages.
At the first stage, the nature of the convulsive syndrome was assessed. To that end, laboratory animals, depending on the convulsive agent used, were divided into four groups and a control group with the introduction of 0.9% sodium chloride solution. Each group consisted of 20 heads. The simulation of convulsive syndrome in the first group was carried out using a reversible acetylcholinesterase inhibitor from the carbamate group, namely phenyl ether of carbamic acid (hereinafter phenylcarbamate) 1 mg/kg bw. The original compound was synthesized at the Golikov Research Center of Toxicology under the leadership of A.Ya. Bespalov. This compound is protected by the patent of the Russian Federation [13]. In the second and third groups, the following were used as model toxicants, respectively: corazol (Pentylentetrazol, 6,7,8,9-tetrahydro-5H-tetrazolo(1,5-a)azepine, C6H10N4), produced by Sigma-Aldrich, at a dose of 65 mg/kg bw and Thiosemicarbazide (Hydrazinecarbothioamide, CH5N3S) at a dose of 8 mg/kg bw, resynthesized in the synthesis laboratory of the Golikov Research Center of Toxicology [14]. In each experimental group, 10 animals were used to study motor activity, neuromotor functions, and the cardiovascular system; 10 animals were used to assess cognitive functions.
The severity of convulsive syndrome after administration of toxicants was determined visually using the upgraded Racine scale [15].
Motor activity and anxiety were assessed using a computerized Open Field test developed by C.S. Hall (1936) [16] using the VideoMot2 system, TSE, Germany. The behavior was evaluated after 24 and 48 h. Seven components of behavior were recorded during 2 min of observation: horizontal and vertical (rack) activity, grooming, the speed of movement of animals, and the distance traveled by the animal during the experiment, total motor activity, the number of movements in the center of the site and on the periphery.
The study of neuromotor functions, clinically expressed by general weakness and asthenia, was carried out after 24, 48 h using a Bioseb GS3 grip analyzer, which automatically registers the gripping force of the lattice with the front paws of the rat and the exact moment of its release.
Cognitive functions were assessed by recording the parameters of the conditioned avoidance responses (CAR) 2, 24, and 48 h after training in a two-compartment PACS-30 installation (Columbus Instruments, USA). The following parameters were recorded: the time of the first entry into the dark compartment (before training to assess the presence of a mink reflex in an animal), the latent period of entering a dark punishable compartment, the time spent in the light and dark compartments. The total duration of the experiment for each animal was 120 seconds. Along with the time parameters of the CAR, in each group, during repeated tests, the number of trained animals was recorded, in which the latent period of entering the dark compartment was more than 120 seconds (the duration of animal observation).
To assess the activity of the cardiovascular system, an electrocardiographic examination (ECG) was performed in the II lead on a Poly-Spectrum-8B electrocardiograph veterinary device (Neurosoft, Russia) after 24 and 48 h. The measured parameters included heart rate (HR), the value of the R wave, the intervals PQ and QT, calculated according to the II standard lead. The heart rhythmogram was evaluated using the Baevsky cardiointervalography (KIG) on the same device [17].
In animals subjected to planned euthanasia and necropsy, a histological analysis of the brain was performed at the first stage of the experiment. The organ sections were dehydrated, impregnated with paraffin and stained with hematoxylin and eosin, followed by light microscopy on a Leica DM1000 microscope, Leica Microsystems Wetzlar GmbH (Germany) at 400x magnification. CO2 inhalation using Open Science equipment (Russia) was used for euthanasia.
At the second stage of the experiment, some biochemical parameters were evaluated, as well as the ionic composition of animal blood. In the phenylcarbamate group, the antioxidant status was additionally assessed in comparison with the intact group. To that end, a new sample was formed in which the convulsive syndrome was modeled with phenylcarbamate at a dose of 1 mg/kg bw, corazol at a dose of 65 mg/kg bw, thiosemicarbazide at a dose of 8 mg/kg bw Depending on the time of blood collection (24 and 48 h; on days 7 and 14), laboratory animals were divided into four groups for the control of the studied parameters, including an intact group of 24 heads each.
Blood from animals for biochemical analysis was collected in a dry container after 24 and 48 h, on days 7 and 14. Next, the selected biological material was centrifuged (centrifuge Z 326 K, manufactured in Germany, series 66110159) at 3000 rpm, at 4°C for 10 min. For further studies, the infusion fluid, serum, was selected. A transparent serum without signs of hemolysis was examined. Biochemical parameters (triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (alkaline phosphatase), total cholesterol, urea, total bilirubin) were determined using a biochemical analyzer A-25 from BioSystems (Spain) using kits from Vector-Best JSC (Russia). The analyzer calibration and internal quality control of the studies were carried out on calibration and control materials of Vector-Best JSC (Russia).
To study the antioxidant system, blood plasma and erythrocyte suspension were used, obtained by centrifugation of whole blood at 3000 g for 3 min, followed by triple washing of erythrocytes with a saline solution and step-by-step centrifugation at the specified parameters. Hemolysate was prepared from the washed erythrocytes in an appropriate manner for each technique. In erythrocyte hemolysate, indicators of the antioxidant protection system were determined using the Habig WH and Jakoby WB method [19]: superoxide dismutase (SOD) [18], glutathione peroxidase (GP), glutathione reductase (GR). To assess the processes of lipid peroxidation (LPO), the concentration of reduced glutathione (RG) was determined [20], as well as the stable end product of LPO — malondialdehyde (MDA) [21]. The study was conducted on an A-25 biochemical analyzer (BioSystems SA).
At the third stage, to study the mechanism of action of phenylcarbamate, the activity of cholinesterase in the blood and brain was evaluated. Acetylcholinesterase activity was determined by the Ellman method [22]. The experimental animals were divided into two groups: the control in the number of 30 heads and the experimental in the number of 30 heads. Male rats from the experimental group were intraperitoneally injected with phenylcarbamate at a dose of 1 mg/kg bw. Animals from the control group were injected intraperitoneally with 0.9% sodium chloride solution. After decapitation, blood and brain tissues were taken from the studied groups at certain time intervals: 10, 30, 60 min, 6 and 24 h. Six animals were involved at one time point.
Statistical processing of the experimental data obtained was carried out using the Statistica v.10 statistical analysis software. A parametric analysis of variance (ANOVA) was used to assess the reliability of differences between the groups in functional and biochemical studies. The nonparametric Mann–Whitney criterion was used to assess the reliability of differences in the dynamics of changes in acetylcholinesterase activity in the brain and in the blood of rats.
RESULTS
The main characteristics of the convulsive syndrome observed with intraperitoneal administration of phenylcarbamate at a dose of 1 mg/kg bw, in comparison with the effects observed following administration of corazol and thiosemicarbazide (TSC), are presented in Table 1.
Table 1. Comparative assessment of the latency period and duration of convulsive syndrome of the studied convulsants
№ | Parameter | PC, 1 mg/kg bw n = 20 | Corazol, 65 mg/kg bw n = 20 | TSC, 8 mg/kg bw n = 20 |
1 | The latent period of seizures, min | 5.33 ± 0.33 | 8.11 ± 0.6 | 106.8 ± 7.34 |
2 | Duration of convulsive syndrome at level 5 according to Racine, min | 30 ± 0.6 | 35 ± 1.9 | 129 ± 9.0 |
3 | Duration of convulsive syndrome at Racine level 6, min | 13 ± 1.5 | 12 ± 1.0 | 150 ± 7.1 |
Table prepared by the authors based on their own data
Note: the data is presented as the average value and the standard error of the average (М ± m).
The time of onset of the latent period of seizures in animals from the group receiving phenylcarbamate at a dose of 1 mg/kg bw is comparable to a similar parameter in male rats receiving corazol at a dose of 65 mg/kg bw (Table 1). A longer latent period of seizure appearance, manifested in the form of both clonic and tonic seizures, as well as extensions, was noted only in animals from the group that received thiosemicarbazide. In the group of animals using phenylcarbamate, the intensity of Racine level 5 convulsive syndrome was recorded in 60% of animals comparable to animals from the comparison groups (the use of corazol and thiosemicarbazide). At the same time, the intensity of Racine level 6 convulsive syndrome was recorded in animals from the groups with corazol and thiosemicarbazide models to a greater extent. It should be noted that the duration of convulsive syndrome at Racine levels 5 and 6 in animals from the group with the phenylcarbamate model was comparable to the data in animals from the group with the corazol model of convulsive syndrome. At the same time, in animals from the group with the thiosemicarbazide model, the duration of convulsive syndrome at both Racine levels 5 and 6 was much longer. In addition, the mortality rate in the phenylcarbamate and corazol groups was 20%, compared to 33% in the thiosemicarbazide group.
When evaluating behavior and motor activity using a multi-purpose open field system, it was found that animals treated with phenylcarbamate had a statistically significant two-fold inhibition of motor activity parameters 24 h after administration compared to the control group of animals. In addition, when corazol was administered, a statistically significant increase in the total motor activity by 2.4 times, the number of horizontal movements by 4.3 times, and grooming acts by 2 times was observed compared to the control. In rats treated with thiosemicarbazide, a statistically significant increase in the total motor activity was revealed by 1.2 times, the number of horizontal movements by 1.9 times, the number of racks by 3.1 times, grooming acts by 1.9 times, average distance by 2.5 times, average speed by 2.6 times, and motor activity on the periphery by 6.6 times.
No statistically significant differences were revealed between grip strength indicators in the animals receiving convulsive doses of phenylcarbamate or other toxicants compared to the control group.
An assessment of cognitive impairment in a CAR test found that animals receiving phenylcarbamate in a convulsive dose registered an increase in the time of the first entry into the dark compartment before training. Thiosemicarbazide showed no statistically significant effect on learning both when the toxicant was administered a day before training and when administered a day after training. Corazole, administered 24 h before training, according to the results obtained, caused a dose-dependent violation of short-term memory by affecting the process of information fixation (memory trace).
When studying the function of the cardiovascular system, a significant decrease in heart rate (HR) was recorded 48 h after administration of phenylcarbamate (403.0 ± 18.8 versus 484.5 ± 8.7 beats per minute in the control group). When assessing the intensity index according to cardiointervalography data after 24 h, significant differences were noted between the groups receiving phenylcarbamate (47715.2 ± 10714.0 vs. 13889.6 ± 4623.0 CU) and thiosemicarbazide (14814.5 ± 6278.8 vs. 73743 ± 16103.0 CU).
Histological examination of brain tissue samples in animals treated with phenylcarbamate revealed a large number of elongated dark cortical neurons without a clear core boundary 24 h after administration; the nucleolus was not visualized compared to the control group (Fig. 1).
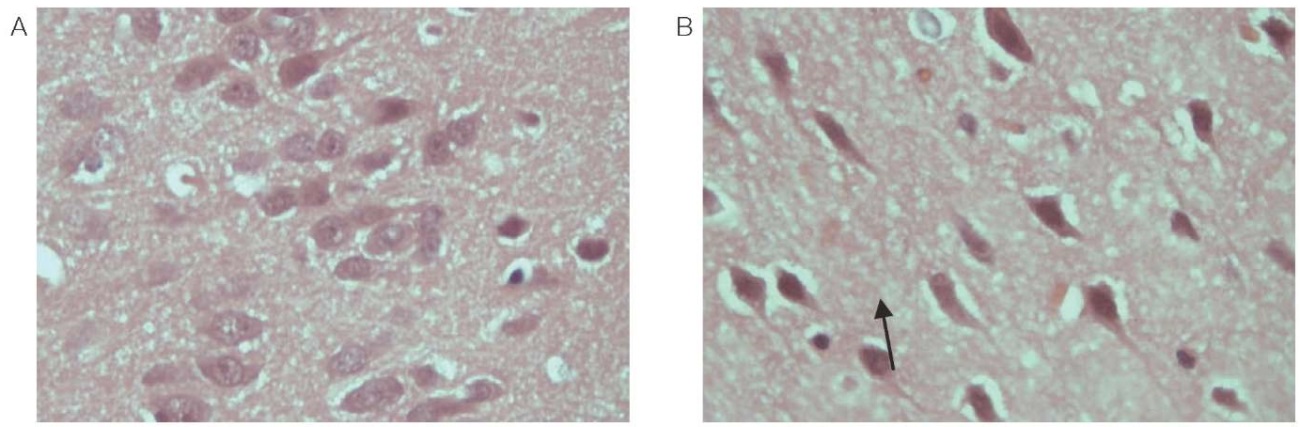
Figure prepared by the authors using their own data
Fig. 1. The cortex of the frontal lobe of the rat brain (magn. ×400)
A is a control animal; B — 24 h after administration of phenylcarbamate.
Note: the arrow indicates a dark elongated neuron without a clear core boundary.
Focal rarefaction of the neuropile was observed in the white matter, mainly due to cell processes (astrocytes, oligodendrocytes, or neurons). The veins in the white matter are stretched, filled with red blood cells that are not stained with eosin well, without clear boundaries. Most neurons of the stem are dark and wrinkled; however, against this background there are individual cells in a state of acute swelling (Fig. 2). In the tissues of the cerebellum, Purkinje cells are dark; the nucleus and nucleolus are not defined. Foci of parenchymal hemorrhage, loss of cells, foci of elective necrosis are observed. The respective data is shown in Fig. 3.
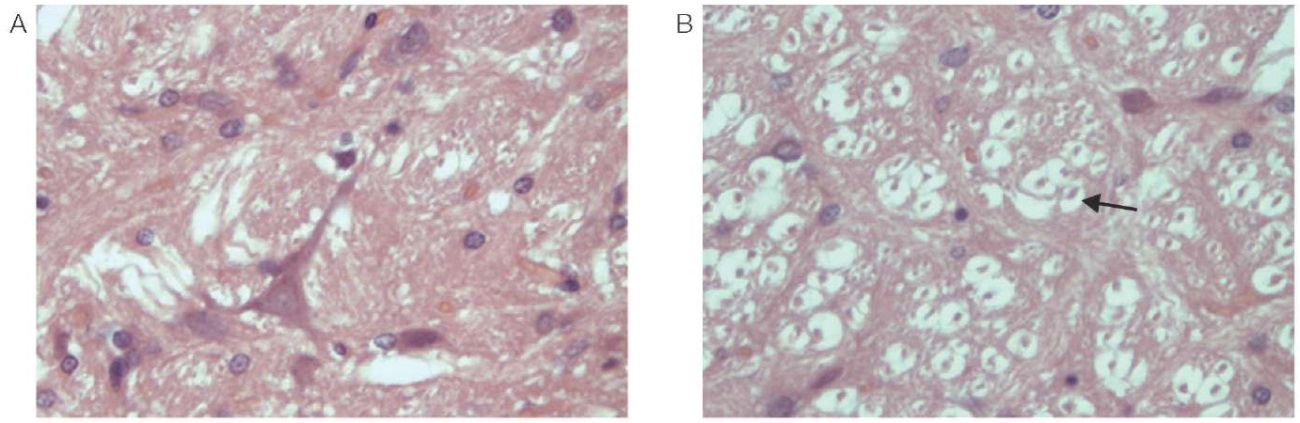
Figure prepared by the authors using their own data
Fig. 2. Rat brain stem (magn. ×400)
A is a control animal; B — 24 h after administration of phenylcarbamate.
Note: the arrow indicates cells in a state of acute swelling.
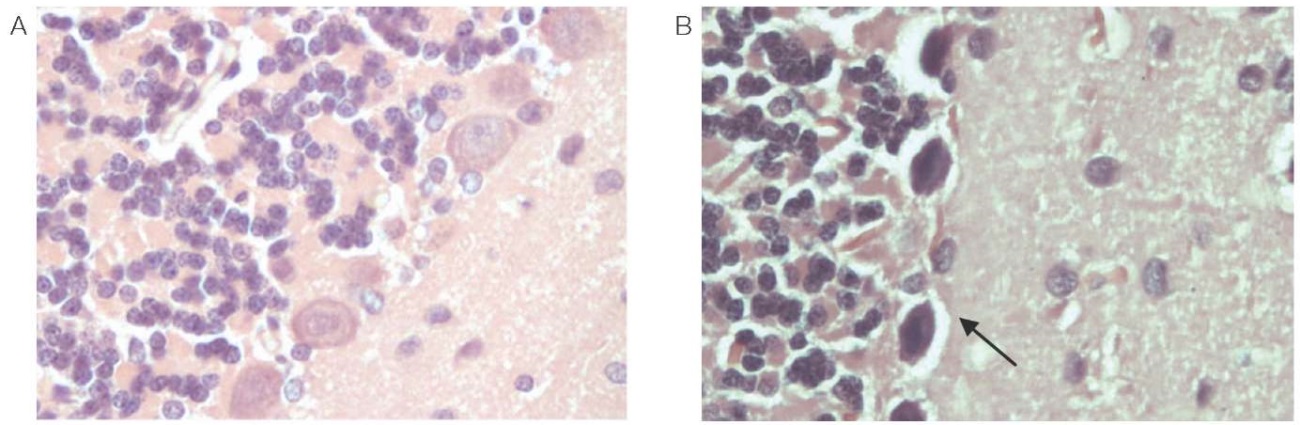
Figure prepared by the authors using their own data
Fig. 3. Rat cerebellum (magn. ×400)
A is a control animal; B — 24 h after administration of phenylcarbamate.
Note: the arrow indicates dark Purkinje cells, the nucleus and nucleolus are not defined.
It is noteworthy that phenylcarbamate, which does not lead to the development of pronounced disorders of motor activity and cognitive functions in the first 24 h after administration (unlike the toxicants of the comparison groups, i.e., corazol and thiosemicarbazide), when evaluated in open field and CAR tests, also negatively affects a number of biochemical parameters after two weeks after exposure. At the same time, after exposure to corazole, individual and less pronounced similar changes were observed.
The data on the biochemical composition of the blood of experimental animals for 24 h and for a longer period are presented in Table 2. After exposure to phenylcarbamate, an increase in triglycerides was recorded in the first days, whose level normalized by the end of the experimental observation period. Some of the changes occurred according to the type of decompensation: an excessive increase in the concentration of a biochemical indicator and its subsequent sharp decrease in response to exposure to a toxicant. At the same time, on the 14th day, a decrease in the average values was revealed for such indicators as ALT, LDH. In addition, on the 14th, day an increase in alkaline phosphatase, urea, and bilirubin, as well as a decrease in cholesterol levels were observed.
Table 2. Effect of the studied convulsive toxicants on biochemical parameters in the blood of animals in different time periods
Test characteristic | Observation period | Intact group n = 24 | PC, 1 mg/kg bw n = 24 | Corazol, 65 mg/kg bw n = 24 | TSC, 8 mg/kg bw n = 24 |
Triglycerides, mmol/L | 24 h | 0.32 ± 0.03 | 0.52 ± 0.05* | 0.45 ± 0.06 | 0.62 ± 0.04* |
48 h | 1.07 ± 0.16 | 1.24 ± 0.17 | 0.68 ± 0.06* | 0.96 ± 0.08 | |
7 days | 1.43 ± 0.26 | 0.87 ± 0.08 | 1.12 ± 0.10 | 1.32 ± 0.20 | |
14 days | 0.83 ± 0.07 | 1.09 ± 0.14 | 1.32 ± 0.13* | 0.76 ± 0.09 | |
Lactate dehydrogenase, U/L | 24 h | 1001.3 ± 114.9 | 916.4 ± 112.6 | 870.8 ± 89 | 754.6 ± 32.3 |
48 h | 909.2 ± 78.6 | 894.2 ± 55.2 | 792.4 ± 52.5 | 867 ± 62.4 | |
7 days | 894.4 ± 61.2 | 816.1 ± 83.3 | 1046.9 ± 79.9 | 929.9 ± 71.5 | |
14 days | 935.7 ± 67.3 | 686.8 ± 63.2* | 759.4 ± 81.3 | 783.7 ± 67.4 | |
Alanine-aminotransferase, U/L | 24 h | 52.8 ± 2.2 | 56.4 ± 5.4 | 56.4 ± 2.3 | 62.5 ± 5.4 |
48 h | 60.7 ± 3.1 | 62.1 ± 2.7 | 65.0 ± 4.4 | 58.4 ± 2.7 | |
7 days | 71.4 ± 2.5 | 69.2 ± 3.9 | 79.4 ± 7.1 | 70.8 ± 4.1 | |
14 days | 55.5 ± 3.2 | 43.6 ± 2.1* | 48.3 ± 1.6 | 53.3 ± 6.2 | |
Aspartate aminotransferase, U/L | 24 h | 132.8 ± 7.6 | 156.6 ± 12.7 | 146.0 ± 5.1 | 146.5 ± 7.7 |
48 h | 143.5 ± 8.3 | 153.4 ± 5.2 | 150.7 ± 7.9 | 155.2 ± 7.4 | |
7 days | 169.7 ± 7.9 | 160.9 ± 12.2 | 169.1 ± 10.9 | 170,9 ± 10.7 | |
14 days | 140.3 ± 7.4 | 142.7 ± 6.7 | 125.7 ± 6.9 | 150.3 ± 14.4 | |
Alkaline phosphatase, U/L | 24 h | 215.8 ± 12.5 | 179.6 ± 11* | 198.1 ± 15.3 | 277.3 ± 20.4* |
48 h | 292.0 ± 22.8 | 328.1 ± 15.9 | 256.2 ± 25.4 | 258 ± 29.3 | |
7 days | 302.7 ± 27.9 | 269.7 ± 23.2 | 305.3 ± 34.3 | 343 ± 25.8 | |
14 days | 210.4 ± 18.7 | 306.5 ± 24.7* | 301.9 ± 22* | 295 ± 46.1 | |
Cholesterol, mmol/L | 24 h | 1.61 ± 0.05 | 1.40 ± 0.08* | 1.22 ± 0.08* | 1.48 ± 0.08 |
48 h | 1.14 ± 0.05 | 1.15 ± 0.06 | 1.15 ± 0.05 | 1.24 ± 0.05 | |
7 days | 1.10 ± 0.08 | 1.03 ± 0.04 | 1.24 ± 0.07 | 1.30 ± 0.08 | |
14 days | 1.61 ± 0.07 | 1.41 ± 0.08* | 1.30 ± 0.07* | 1.45 ± 0.08 | |
Total bilirubin, µmol/L | 24 h | 10.2 ± 0.4 | 12.0 ± 1.0 | 12.1 ± 1.3 | 14.1 ± 1.5* |
48 h | 18.2 ± 2.8 | 20.2 ± 1.8 | 10.5 ± 1.6* | 17.6 ± 1.3 | |
7 days | 13.8 ± 2.8 | 14.4 ± 2.3 | 19.6 ± 2.1 | 17.9 ± 2.0 | |
14 days | 12.2 ± 1 | 17.6 ± 1.8* | 18.2 ± 2.7* | 14.3 ± 1.3 | |
Urea, mmol/L | 24 h | 4.4 ± 0.2 | 3.6 ± 0.3* | 4.0 ± 0.3 | 4.7 ± 0.3 |
48 h | 4.5 ± 0.3 | 5.9 ± 0.3* | 4.7 ± 0.6 | 4.2 ± 0.1 | |
7 days | 5.2 ± 0.5 | 5.4 ± 0.2 | 5.4 ± 0.2 | 6.4 ± 0.4 | |
14 days | 4.2 ± 0.2 | 5.6 ± 0.3* | 4.9 ± 0.2* | 3.9 ± 0.3 |
Table compiled by the authors using their own data
Note: the data is presented as the mean and the standard error of the mean (M ± m).
* — confidence probability of differences relative to the control group (p < 0.05).
The evaluation of the ionic composition of blood (Table 3), characterizing, among other things, kidney function, found that the changes caused by phenylcarbamate on the first day (a decrease in the concentration of sodium and magnesium ions) are similar to those observed after exposure to thiosemicarbazide. This may indicate excessive ion consumption at the time of convulsive syndrome.
Table 3. The effect of the studied convulsive toxicants on the ionic composition of animal blood in various time periods of convulsive syndrome
Test characteristic | Observation period | An intact group n = 24 | Corazol, 65 mg/kg bw n = 24 | TSC, 8 mg/kg bw n = 24 | PC, 1 mg/kg bw n = 24 |
K+, mmol/L | 24 h | 4.7 ± 0.1 | 4.5 ± 0.1 | 4.6 ± 0.1 | 4.9 ± 0.1 |
48 h | 4.6 ± 0.2 | 4.6 ± 0.1 | 4.6 ± 0.1 | 4.8 ± 0.1 | |
7 days | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.0 ± 0.1 | 4.2 ± 0.1 | |
14 days | 5.2 ± 0.2 | 5.1 ± 0.1 | 5.1 ± 0.2 | 5.7 ± 0.2 | |
Na+, mmol/L | 24 h | 160 ± 0.9 | 153.6 ± 1.5* | 157.6 ± 1.4 | 155.4 ± 1.2* |
48 h | 155.3 ± 1.3 | 157.7 ± 1.7 | 158.6 ± 1.2 | 159.0 ± 1.0* | |
7 days | 162.1 ± 1.3 | 161.1 ± 1.5 | 163.5 ± 1.0 | 162.7 ± 1.2 | |
14 days | 145.2 ± 1.6 | 142.9 ± 1.9 | 143 ± 1.7 | 142.9 ± 1.9 | |
CI–, mmol/L | 24 h | 96.6 ± 1.5 | 95.1 ± 1.1 | 99 ± 1.6 | 99.2 ± 1.1 |
48 h | 96.9 ± 1.0 | 94.8 ± 1.7 | 96.6 ± 0.7 | 99.2 ± 1.2 | |
7 days | 93.6 ± 1.5 | 92.6 ± 1.6 | 92.9 ± 1.2 | 94.4 ± 1.1 | |
14 days | 93.8 ± 0.7 | 92.5 ± 1.4 | 92.3 ± 1.7 | 96.5 ± 1.1* | |
Phosphorus (Р), mmol/L | 24 h | 2.89 ± 0.07 | 2.82 ± 0.08 | 2.64 ± 0.07* | 2.85 ± 0.08 |
48 h | 2.65 ± 0.05 | 2.69 ± 0.10 | 2.49 ± 0.03* | 2.94 ± 0.06* | |
7 days | 2.14 ± 0.07 | 2.12 ± 0.05 | 2.14 ± 0.06 | 2.29 ± 0.07 | |
14 days | 2.85 ± 0.08 | 2.68 ± 0.06 | 2.65 ± 0.11 | 2.63 ± 0.04* | |
Magnesium (Mg), mmol/L | 24 h | 1.37 ± 0.25 | 0.35 ± 0.07* | 0.94 ± 0.01 | 0.27 ± 0.02* |
48 h | 1.23 ± 0.19 | 0.89 ± 0.02 | 0.96 ± 0,02 | 0.90 ± 0.02 | |
7 days | 1.27 ± 0.19 | 0.88 ± 0.03 | 1.19 ± 0.18 | 0.90 ± 0.02 | |
14 days | 0.56 ± 0.03 | 0.63 ± 0.02 | 0.57 ± 0.05 | 0.67 ± 0.02* |
Table compiled by the authors using their own data
Note: the data is presented as the mean and the standard error of the mean (M ± m).
* — confidence probability of differences relative to the control group (p < 0.05).
Since phenylcarbamate is an acetylcholinesterase (AChE) inhibitor, it was necessary to assess the level of its effect on the activity of AChE in the developed model of convulsive syndrome. Table 4 shows the results of determining the AChE level in whole blood and the percentage of inhibited AChE in the blood and brain after administration of phenylcarbamate in a convulsive dose.
Table 4. Dynamics of changes in acetylcholinesterase activity in the brain and blood of white rats after administration of phenylcarbamate
Time | PC (n = 6) | Control (n = 6) | % inhibition, blood | % inhibition, brain | ||
blood AChE, U/mL | brain AChE, U/mg | blood AChE, U/mL | brain AChE, U/mg | |||
10 min | 463.1 [ 365.4; 480.5]* | 35.1 [ 32.7; 38.5]* | 757.4 [ 606.4; 796.4] | 78 [ 70.7; 80.4] | 38.9 [ 36,6; 51,8] | 54.9 [ 50.6; 58.1] |
30 min | 370.9 [ 328.8; 396.2]* | 66.3 [ 59.9; 79.7]* | 563.7 [ 556.9; 634.9] | 88.4 [ 87; 92.7] | 34.2 [ 29.7; 41.7] | 25.1 [ 9.9; 32.3] |
60 min | 441.1 [ 292.1; 494.7] | 63.4 [ 51.3; 70.6] * | 690.1 [ 469.2; 700.0] | 92 [ 90.6; 104.5] | 36.1 [ 28.3; 57.7] | 31.0 [ 23.2; 44.2] |
6 h | 492.4 [ 418.8; 543.8] | 68.1 [ 55.0; 86.2] | 698.7 [ 686.8; 777.6) | 120.3 [ 108; 144.6] | 29.5 [ 22.2; 56.9] | 43.4 [ 28.3; 54.3] |
24 h | 946.3 [ 398.6; 996.6] | 123.9 [ 111.8; 134.6] | 763.6 [ 629.8; 798.3] | 117.3 [ 109.9; 126.3] | -23.9 [ -30.5; 47.8] | -5.6 [ -14.7; 4.8] |
Table compiled by the authors using their own data
Note: the data is presented as median (Me), upper (UQ), and lower (LQ) quartiles.
* — confidence probability of differences relative to the control group (p < 0.05).
An analysis of the data presented in Table 4 revealed significant inhibition of AChE in the brain and in the whole blood of white rats within 6 h after administration of phenylcarbamate. After 24 h, the enzyme activity was completely restored. It is important to note that 6 h after administration of phenylcarbamate, with continued inhibition of AChE by 42.3% and 30.5% in the central nervous system and peripheral blood, respectively, seizures in rats completely stopped.
Additionally, the state of individual components of the antioxidant system and markers of lipid peroxidation in different time periods after exposure to phenylcarbamate at a dose of 1 mg/kg bw was studied.
It was found that on the first day after administration of phenylcarbamate to animals, three of the four studied parameters changed significantly. At the same time, the level of malondialdehyde in the phenylcarbamate group on the first day of observation was reduced by almost twofold compared to the control group. On the 14th day of observation, its level significantly exceeded the control indicators; at the same time, the activity of glutathione peroxidase showed the opposite direction of changes compared to the control (Table 5).
Table 5. Effect of phenylcarbamate on the level of malondialdehyde and indicators of the antioxidant system in the blood of laboratory animals
Experimental groups | Observation period | Test characteristic | |||
RG, µmol/L | MDA, nmol/mL | SOD, U/g Hb | GP, U/g Hb | ||
Control | 24 h | 0.28 ± 0.02 | 142.3 ± 8.3 | 1131.0 ± 51.3 | 34.7 ± 1.3 |
PC | 0.36 ± 0.02* | 79.6 ± 10.9* | 1331.1 ± 70.8 | 42.5 ± 2.7* | |
Control | 48 h | 0.13 ± 0.04 | 188.3 ± 11.8 | 1274.9 ± 141.2 | 27.7 ± 2.4 |
PC | 0.21 ± 0.03 | 151.3 ± 4.2* | 1494.5 ± 194.1 | 33.7 ± 3.9 | |
Control | 7 days | 0.41 ± 0.06 | 122.6 ± 3.9 | 1297.9 ± 76.6 | 35.8 ± 1.0 |
PC | 0.40 ± 0.04 | 153.1 ± 9.7* | 1096.7 ± 81.0 | 32.3 ± 1.6 | |
Control | 14 days | 0.39 ± 0.03 | 164.0 ± 12.2 | 1702.8 ± 66.9 | 35.6 ± 1.1 |
PC | 0.34 ± 0.07 | 197.6 ± 8.2* | 1504.8 ± 59.7 | 31.6 ± 1.3* | |
Table compiled by the authors using their own data
Note: the data is presented as the mean and the standard error of the mean (M ± m).
* — confidence probability of differences relative to the control group (p < 0.05).
DISCUSSION
Due to their specific characteristics, models using OP-based cholinesterase inhibitors show a greater promise for the development of antidotes than models for testing antiepileptic drugs [10]. According to our findings, the action of phenylcarbamate on the body of experimental animals have a number of specific features that should be considered when both studying the pathogenesis of poisoning and developing respective treatment approaches. The study of the key characteristics of convulsive syndrome in acute poisoning for 2 h demonstrated that, in general, the phenylcarbamate-based model (intraperitoneal administration) is comparable to the corazole-based screening convulsive model common in preclinical research practice. When using phenylcarbamate as a model toxicant, the intensity of seizures was slightly less pronounced (level 5 in >60% of animals) than that in the comparison groups (level 6 intensity with the administration of corazol and thiosemicarbazide). It is important to note that the severity of seizures of level 5 on the Racine scale corresponds to generalized convulsive syndrome in humans, which allows us to recommend phenylcarbamate as an effective toxicant for modeling severe convulsive syndrome in the development of therapeutic treatments.
The revealed changes in heart rate indicate that developers of carbamate poisoning treatments should pay attention to drugs that affect the functions of the heart and blood vessels. At the same time, the developed model based on the administration of phenylcarbamate can serve as a basis for testing appropriate treatment approaches. In general, the recorded changes in ECG parameters agree with the literature data that described the pronounced effect of carbamates and OP on cardiovascular activity [23].
Standard tests for assessing motor activity and cognitive impairment are low-informative when used on the first day after exposure to phenylcarbamate. Therefore, it can be assumed that disorders of the nervous system developing after such poisoning have a long and complex pathogenesis, requiring either longer observations or the use of alternative tests in experimental modeling of poisoning with carbamate compounds.
The conducted histological examination of brain tissues confirm that, despite the absence of pronounced deviations in tests for assessing cognitive impairment in experimental animals in the first 24 h after exposure, the introduction of carbamates lead to disorders in the structure of the brain. This information is relevant both to studies into the pathogenesis of poisoning and to the practice of preclinical research in the development of treatment approaches to poisoning.
Some of the revealed changes in biochemical parameters may be associated not only with a set of pathogenetic transformations during intoxication, but also with the individual sensitivity of subjects. In our study, the groups were limited in number, which might have affected the average values of indicators. However, the results of our study are confirmed by scientific data obtained when evaluating the effect of other cholinesterase inhibitors on humans. Thus, a study by Senarathne R. et al. proposed to use AST and ALT as alternative markers of carbamate and OP poisoning [24]. The revealed changes in the activity of liver enzymes, cholesterol, and triglycerides 14 days after exposure to phenylcarbamate indicate a violation of liver function. An increase in the concentration of urea in the blood can be associated with both impaired liver function and damage to muscle tissues. In addition, in the process of massive protein breakdown accompanied by hyperammonemia, toxic ammonia can cause the development of a number of neurological disorders.
When exposed to phenylcarbamate, along with magnesium deficiency, the parameters of heart rate changed. Therefore, it can be assumed that these changes contribute to convulsive activity independent of interaction with AChE, given that hypomagnesemia is frequently associated with myocardial hyperexcitability, tremor, and fasciculations.
The results of studying the content and function of AChE with the administration of phenylcarbamate are quite expectable, confirming the effectiveness of using this compound as a model toxicant in experimental modeling of convulsive syndrome in the setting of poisoning with cholinesterase inhibitors.
The studied markers of lipid peroxidation and those of the antioxidant protection system confirmed the data on the complex mechanism of the development of seizures and subsequent neurological disorders after carbamate poisoning. The previously detected change in the level of malondialdehyde, formed during lipid peroxidation and associated with changes in the lipid profile [25], correlates with the changes in triglyceride and cholesterol concentrations were established in our study. There were differences in the level of MDA for the first day in the phenylcarbamate group compared to the control group. However, starting from the second day and up to the 14th day, an increase in this indicator was observed compared to the control group. An increase in the levels of GP and RG on the 1st and 2nd days of observation was noted in the group of animals treated with phenylcarbamate, compared to the control group. This confirms impaired liver function and cardiovascular system, as well as the development of an inflammatory reaction in the setting of carbamate poisoning, accompanied by activation of antioxidant mechanisms.
Generalizing the results of our study into the development of an experimental model of convulsive syndrome based on the introduction of phenylcarbamate into the body, the following algorithm of its use can be proposed (see Table 6).
Table 6. Algorithm for the development of an experimental model of convulsive syndrome with a reversible acetylcholinesterase inhibitor
Type of animals | White mongrel male rats aged 3 months, weighing 150–250 g |
Number of animals in the group | At least 6 |
Model toxicant | Phenyl ether of carbamic acid [13] |
Minimum convulsive dose | 1 mg/kg bw (0.9% sodium chloride solution as a solvent) |
Method for introducing the toxicant into the body | Intraperitoneal |
Significant characteristics of convulsive syndrome | Clonic seizures in 100% of cases; latent period of seizures 5–6 min; the intensity of seizures is at least 5 points on the Racine scale for 30 min |
When studying the effectiveness of convulsive syndrome therapy (up to 24 h), additional indicators may be taken into account | Elevated levels of triglycerides, reduced glutathione, and glutathione peroxidase activity Reduced concentrations of cholesterol, alkaline phosphatase, urea, Na+, Mg2+, malondialdehyde; Decrease in heart rate |
When studying the effectiveness of therapies for the long-term effects of convulsive syndrome (up to 14 days), additional indicators may be taken into account | Increase in alkaline phosphatase, urea, bilirubin, malondialdehyde Reduction of cholesterol, lactate dehydrogenase, ALT, glutathione peroxidase |
Table compiled by the authors using their own data
CONCLUSION
The developed experimental model of convulsive syndrome based on phenylcarbamate at a dose of 1 mg/kg bw has demonstrated acceptable characteristics compared to existing models. Therefore, this model can be recommended for preclinical studies when studying the pathogenesis of carbamate poisoning, developing means of relieving convulsive syndrome in acute poisoning, and developing approaches to prevention and treatment of long-term consequences of poisoning in survivors.
Authors’ contributions. All authors confirm that their authorship meets the ICMJE criteria. The greatest contribution is distributed as follows: Aleksandra S. Melekhova — development and testing of an experimental model, writing sections of the article; Alisa V. Belskaya — development and testing of an experimental model, writing sections of the article; Veronika N. Zorina — review of scientific literature, interpretation of the results of biochemical analysis, writing the article; Margarita V. Melnikova — testing of an experimental model; Larisa G. Kubarskaya — determination of acetylcholinesterase; Olga N. Gaikova — histological examination.
References
1. Ghosh AK, Brindisi M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2020;63:2751–88. https://doi.org/10.1021/acs.jmedchem.9b01541
2. Matosevic A, Bosak A. Carbamate Group as Structural Motif in Drugs: a review of carbamate derivatives used as therapeutic agents. Arhiv za higijenu rada i toksikologiju. 2020;71(4):285–99. https://doi.org/10.2478/aiht-2020-71-3466
3. King AM, Aaron CK. Organophosphate and carbamate poisoning. Emerg Med Clin North Am.2015;33(1):133–51. https://doi.org/10.1016/j.emc.2014.09.010
4. Mangaly AJ, Radhakrishnan C. Alternate Biochemical Markers in Organophosphate Poisoning. J Assoc Physicians India. 2023;71(8):11–2. https://doi.org/10.59556/japi.71.0325
5. Morgan JE, Wilson SC, Travis BJ, Bagri KH, Pagarigan KT, Belski HM, et al. Refractory and Super-Refractory Status Epilepticus in Nerve Agent-Poisoned Rats Following Application of Standard Clinical Treatment Guidelines. Front Neurosci.2021;15:732213. https://doi.org/10.3389/fnins.2021.732213
6. Alozi M, Rawas-Qalaji M Treating organophosphates poisoning: management challenges and potential solutions. Crit Rev Toxicol.2020;50(9):764–79. https://doi.org/10.1080/10408444.2020.1837069
7. Leung MCK, Meyer JN. Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies. Reprod Toxicol. 2019;89:83–92. https://doi.org/10.1016/j.reprotox.2019.07.007
8. Mudyanselage AW, Wijamunige BC, Kocon A, Carter WG. Differentiated Neurons Are More Vulnerable to Organophosphate and Carbamate Neurotoxicity than Undifferentiated Neurons Due to the Induction of Redox Stress and Accumulate OxidativelyDamaged Proteins. Brain Sci. 2023;13(5):728. https://doi.org/10.3390/brainsci1305072
9. Löscher W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem Res. 2017;42(7):1873–88. https://doi.org/10.1007/s11064-017-2222-z
10. McCarren HS, McDonough JH Jr. Anticonvulsant discovery through animal models of status epilepticus induced by organophosphorus nerve agents and pesticides. Ann N Y Acad Sci. 2016;1374(1):144–50. https://doi.org/10.1111/nyas.13092
11. СанПиН 3.3686-21 Санитарно-эпидемиологические требования по профилактике инфекционных болезней. СанПиН 3.3686-21 Санитарно-эпидемиологические требования по профилактике инфекционных болезней.
12. Обоснование и разработка порядка применения наиболее эффективных препаратов, предлагаемых в качестве средств фармакологической коррекции последствий отравлений веществами судорожного действия. Отчет о НИР (заключительный). ФГБУН ИТ ФМБА России, рук. Петров А.Н., исполн.: Войцехович К.О. и др.СПб.; 2017 г. № 115021340031. Обоснование и разработка порядка применения наиболее эффективных препаратов, предлагаемых в качестве средств фармакологической коррекции последствий отравлений веществами судорожного действия. Отчет о НИР (заключительный). ФГБУН ИТ ФМБА России, рук. Петров А.Н., исполн.: Войцехович К.О. и др.СПб.;2017 г. № 115021340031.
13. Bespalov AYa, Prokopenko LI, Gorchakova TL, Kozlov VK, Petrov AN, Zaitseva MA, et al. Hydrochlorides of substituted 2-[(dimethylamino)methyl] aryldimethyl carbomates with anticholinesterase activity. Patent of the Russian Federation. No. 2754133; 2021 (In Russ.).
14. Mironov AN, ed. Guidelines for conducting preclinical studies of medicinal products. Moscow: Grif i K; 2012 (In Russ.).
15. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94. https://doi.org/10.1016/0013-4694(72)90177-0
16. Hall CS Emotional behavior in the rat. III. The relationship between emotionality and ambulatory activity. J. comp. physiol. Psychol. 1936;22:345–52. https://doi.org/10.1037/h0059253
17. Strutynskij AV. Electrocardiogram. Analysis and interpretation. Мoscow: MEDpress- inform; 2013 (In Russ.).
18. Batotsyrenova EG, Kashuro VA, Sharabanov AV. Pharmacological correction of longterm effects of acute severe poisoning with sodium thiopental under chronic light desynchronosis. Journal Biomed. 2021;17(3):23–8 (In Russ.). https://doi.org/10.33647/2074-5982-17-3-23-28
19. Habig WH. Assay for differentiation of glutathione S-transferases. Methods in Enzymology. 1981;77:398–405. https://doi.org/10.1016/s0076-6879(81)77053-8
20. Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–7. https://doi.org/10.1016/0003-9861(59)90090-6
21. Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry. 1978;86(1):271–8. https://doi.org/10.1016/0003-2697(78)90342-1
22. Ellmann GL, Courtney KD, Andress V, Fcatherstone RM. A new and rapid Colorimetric determination of activity acetylcholinesterase. Biochem.Pharmacol. 1961;7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
23. Yadav I. Study of Sick Euthyroid Syndrome in Organophosphate Poisoning. J Assoc Physicians India. 2022; 70(4):11–2.
24. Senarathne R, Hettiaratchi U, Athiththan L, Peiris H, Sarathchandra C, Senanayake H, Weerawansa P, Siribaddana S. Selected Liver Markers in Predicting the Severity of Organophosphate and Carbamate Poisoning. J Environ Public Health. 2022:7826396. https://doi.org/10.1155/2022/7826396
25. Bulatova IA, SHCHyokotova AP, Karlysheva KN. Features of oxidative stress in metabolic syndrome with fatty liver disease. Modern problems of science and education. 2014:2. URL: https://science-education.ru/ru/article/view?id=12473 (In Russ.).
About the Authors
A. S. MelekhovaRussian Federation
Saint-Petersburg
A. V. Belskaya
Russian Federation
Saint-Petersburg
V. N. Zorina
Russian Federation
Saint-Petersburg
M. G. Melnikova
Russian Federation
Saint-Petersburg
L. G. Kubarskaya
Russian Federation
Saint-Petersburg
O. N. Gaikova
Russian Federation
Saint-Petersburg
Review
For citations:
Melekhova A.S., Belskaya A.V., Zorina V.N., Melnikova M.G., Kubarskaya L.G., Gaikova O.N. Experimental model of convulsive syndrome based on phenylcarbamate. Extreme Medicine. 2024;26(4):38-48. https://doi.org/10.47183/mes.2024-26-4-38-48

















