Scroll to:
Assessment of depression-like behavior in mice after fractional gamma irradiation
https://doi.org/10.47183/mes.2024-26-3-30-39
Abstract
Introduction. Exposure of the brain to high doses of ionizing radiation is an established risk factor for the development of neoplasms and associated cognitive disorders. However, the impact of long-term low-dose irradiation on the brain and higher nervous system, including the development of anxiety-depressive disorders, remains an unsolved problem.
Objective. To study the effect of fractionated gamma-irradiation in doses of 0.1 Gy, 1 Gy and 5 Gy in the early postnatal period on indices of depression-like states in C57Bl/6 mice at the age of 1 and 6 months.
Materials and methods. The animals were irradiated during the first month of life. Cumulative doses (0.1 Gy, 1 Gy and 5 Gy) were obtained in the mode of fractionated irradiation (20 fractions). 2 control groups were formed comprising intact animals and falsely irradiated animals. The presence of a depression-like state was evaluated in the “tail holding” test at the age of 1 month and 6 months.
Results. Age-related changes were manifested by a decrease in depression-like behavior in 6-month-old mice compared with 1-month-old mice. Stress induced by performing radiation-related manipulations, which had no significant effect on 1-month-old mice, led to the development of marked depression-like states in the same animals at 6 months of age. Radiation exposure led to the development of a dose-dependent antidepressant-like effect, which was more pronounced in animals at the age of 6 months after fractionated irradiation at doses of 0.1 Gy and higher.
Conclusions. Fractionated gamma-irradiation does not lead to the development of depression-like symptomatology in mice in the early postnatal period, but, on the contrary, is characterized by antidepressant action.
Keywords
For citations:
Obvintseva N.A., Atamanyuk N.I., Shaposhnikova I.A., Peretykin A.A., Pryakhin E.A. Assessment of depression-like behavior in mice after fractional gamma irradiation. Extreme Medicine. 2024;26(3):30-39. https://doi.org/10.47183/mes.2024-26-3-30-39
INTRODUCTION
It is known that acute irradiation of the brain in high doses leads to cognitive dysfunction, which is generally manifested as a deficit of hippocampus-dependent functions of learning and memory, and that these effects are correlated with the irradiation dose [1–3]. However, despite the considerable body of information on the influence of ionizing radiation on the human body, the formation of mental disorders and the nature of mental disorders in humans depending on the irradiation dose remains under-researched.
In addition to changes in cognitive function, a number of studies have also noted changes in emotional behavior — in particular, the development of signs of anxiety and depression. Thus, a high prevalence of cerebrovascular diseases, organic psychiatric and depressive disorders, cognitive impairment and dementia were noted among the Chernobyl accident liquidators, which cases grew with increasing radiation dose, some effects being registered at doses from 50 mSv [4][5]. An increased incidence of depression and suicidal ideation has been reported among adolescents exposed at an early age to radioactive fallout from the Chernobyl accident [6]. A wide range of depressive pathologies against a background of organic disorders was revealed in persons irradiated in the radiation accidents zone of the Southern Urals [7].
However, when analyzing data on radiation effects in populations irradiated as a result of radiation incidents, it should be considered whether the stress associated with receiving information about possible radiation exposure regardless of whether the background doses were actually exceeded may be responsible for the increased incidence of psychiatric disorders [8].
In order to exclude the effect of factors other than radiation exposure, it is of interest to study the peculiarities of radiation exposure on mental states in experimental animals. Behavioral changes characterized by increased anxiety have been detected in rodents under irradiation at doses in the range of 0.25–1 Gy [9–11]. Dos Santos M et al. registered depressive-like symptoms in animals, which were manifested in the increase of immobility time in the forced swimming (Porsolt) test at brain irradiation in the dentate gyrus region at doses from 0.25 Gy [10]. At the same time, a group of authors Wang H, Ma Z, Shen H, et al. noted depressive-like behavior in rats irradiated at the level of 5 Gy at an early age as manifested in the forced swimming test and tail holding test, which remained the same within 4 months after irradiation [12].
Brain exposure to high doses of ionizing radiation is an established risk factor for the development of neoplasms and cognitive disorders [1]. However, the effect of long-term low-dose irradiation on the central nervous system (CNS) and higher nervous activity remains poorly understood. Long-term exposure due to radiation incidents is characteristic both of emergency professionals and the general population living in the proximate zones. This type of radiation exposure will be additionally characteristic for astronauts during long space flights outside the Earth’s magnetic field (flights to the Moon, Mars, etc.).
It should be noted that the available data on the effects of chronic or fractionated radiation and their impact on the higher nervous activity of experimental animals, especially in the aspect of long-term irradiation at low doses, are contradictory [1][3]. Most studies of the effects of ionizing radiation on mental functions were conducted using a single acute exposure, while clinically and environmentally significant effects on humans occur predominantly in the mode of chronic or fractionated irradiation. Due to the fact that sex differences in depression-related behavior can be registered in humans and mice [13], studies to assess the effect of fractionated irradiation on depression-like behavior in mice should take gender specifics in the implementation of radiation-induced behavioral changes into account.
The present work set out to evaluate the effect of early fractionated irradiation at cumulative doses of 0.1 Gy, 1 Gy and 5 Gy on depression-like behavior in mice of different sexes both immediately after irradiation and over longer periods of time.
МАТЕRIALS AND METHODS
Male and female mice of the C57BL/6 line (SPF-vivarium nursery of Institute of Cytology and Genetics SB RAS, Novosibirsk) were used in this work. The animals were kept under standard vivarium conditions on a standard diet under natural light.
The effect of prolonged radiation exposure in the early postnatal period, which is characterized by the greatest sensitivity of the brain to the action of ionizing radiation, was evaluated at low (0.1 Gy), medium (1 Gy) and high (5 Gy) doses. Starting from 0–3 days after birth and continuing for the first month of life, the animals were subjected to fractionated total external gamma-irradiation 5 days a week for 4 weeks (20 fractions in total).
All experimental animals were divided into groups according to sex and the level of external gamma irradiation:
- 5 Gy group — mice (40 males and 40 females) irradiated at a cumulative dose of 5 Gy (20 fractions of 0.25 Gy each);
- 1 Gy group — mice (40 males and 40 females) irradiated at a cumulative dose of 1 Gy (20 fractions of 0.05 Gy);
- 0.1 Gy group — mice (40 males and 40 females) irradiated at a cumulative dose of 0.1 Gy (20 fractions of 0.005 Gy);
- 0 Gy group — false irradiation (0 Gy) (40 males and 40 females). Animals in this group were treated with similar manipulations and in the same amount as in the “irradiation” groups, but without exposure to ionizing radiation. Stress associated with manipulations included: early postnatal stress in the form of short-term (3–5 min) deprivation from the mother; stress associated with cell transfer, sound, light simulation of irradiation at the IGUR-1M facility;
- Biological control group (BC) — intact animals (40 males and 40 females).
Irradiation was carried out at the IGUR-1M experimental radiobiological unit with 137Cs-sources (CJSC “Kvant”, Russia). The irradiation dose rate at single doses of 0.25 Gy and 0.05 Gy was 0.72 Gy/min; to obtain a single dose of 0.005 Gy, lead collimators were used to reduce the dose rate to 0.015 Gy/min. Gamma field irregularity in the working space was not more than 10%. For irradiation, mice were placed inside the facility in home cages, removing from them lactating females for the duration of irradiation.
On the IGUR-1M unit, five cages with simultaneously irradiated animals were vertically arranged on top of each other. Each day, the position of cells in the row of five was changed. The absorbed dose in each irradiation cycle was monitored using a DKS5350/1 clinical dosimeter (UE “Atomteh”, Republic of Belarus) fitted with a TM31010 cylindrical ionization chamber (PTW-Freiburg, Germany having a volume of 0.125 cm3 in the mode of K(a) kerma measurement in the air of X-ray and gamma radiation. For each cage containing animals, the actual absorbed dose for 20 fractions of irradiation was individually calculated taking into account the dosimeter readings and the calculated value of dose uncertainty.
When calculating the actually received cumulative absorbed dose for animals of each experimental group as based on the DKS5350/1 dosimeter readings in each irradiation cycle and calculated values of standard uncertainties of irradiation doses on the IGUR-1M unit, the following values were obtained for mice: for the 0.1 Gy group — (0.11 ± 0.01) Gy; for the 1 Gy group — (1.00 ± 0.08) Gy; for the 5 Gy group — (5.2 ± 0.4) Gy [14].
At the age of 1 month, 10 individually labelled mice of the same sex were placed in separate cages. Testing was performed at the age of 35–37 days (one week after completion of irradiation and weaning) and again at the age of 6 months (5 months after completion of irradiation). In each experimental group, 80 animals (40 males and 40 females) were tested.
The depression-like state in mice was assessed via the tail-holding test (analog of the Porsolt forced swimming test), which represents the animal’s reaction to a short-term unavoidable stress in the form of immobile suspension (immobility). The test assessed the time, during which the animal switches from active attempts to free itself from an unpleasant position (suspension by the tail) to immobility, which is interpreted as a manifestation of despair behavior and reflects a depression-like state of rodents [15].
To perform the test, mice were suspended by their tails with 15 cm long pieces of painter’s tape from a bar located on two racks. Four animals were tested at a time, separating them from each other with cardboard partitions. Plastic tubes 2/3 the length of the tail attached to the tail to prevent the mouse from being able to climb up it. At least 30 cm was left between the nose of the mouse and the table surface. After suspension, the behavior of the mice was recorded by video (Sony α37 camera) for 6 min, after which the animals were released [15]. The total time during which the mice hung motionless without making active attempts to free themselves (total immobility time); the number of such immobile suspensions; the time until the first immobile suspension; and the average time of one immobile suspension were recorded. The indicators were recorded using the RealTimer program (“Open science” research and production company LLC, Russia).
Data were analyzed in Microsoft Excel and using the R programming language [16]; the results were expressed as mean values and standard errors (M ± SE). We evaluated the conformity of the measured parameters in each group to the normal distribution using the Kolmogorov-Smirnov test of agreement with a statistical significance level of 0.05. Since all analyzed indicators corresponded to normal distribution, the experimental groups were compared using Student’s t-criterion. A significance level of 0.05 was considered reliable. Multivariate variance analysis was performed using a generalized linear model to assess the linear dependence of the studied parameters on the radiation dose, sex, and stress factor associated with manipulations during irradiation of animals (animals from the biological control group were assumed not to experience stress, while animals from the other groups, including the 0 Gy group, were exposed to stress).
RESULTS
In the BC group of mice at 1 month and 6 months of age, all analyzed parameters in mice at 6 months of age had no statistically significant differences between males with the exception of mean time to one immobile suspension (t = 2.33, p = 0.02): time to first immobile suspension at 1 month of age — t = 1.03, p = 0.31; at age 6 months — t = 0.74, p = 0.46); total immobile time at age 1 month — t = 0.20; p = 0.84; at age 6 months — t = 1.41, p = 0.16; number of immobile suspensions in mice at age 1 month — t = 0.17, p = 0.86; at 6 months of age — t = 1.25, p = 0.22; mean time per immobile suspension in mice at 1 month of age — t = 0.86, p = 0.39. In the experimental groups, there were generally no differences between males and females. However, sex differences were observed in animals in the false irradiation group (0 Gy) at 1 month of age: males had shorter time to first immobile suspension (t = 2.13, p = 0.04), as well as a longer total immobile time (t = 2.5, p = 0.01) and mean time to one immobile suspension (t = 2.3, p = 0.02) compared to females. Among the exposed animals, only one group (1 month, 1 Gy) showed differences between females and males in terms of time to first immobile suspension (t = 2.6, p = 0.01), which was shorter in males. Further, the test results in each experimental group were analyzed without sex separation (Table 1).
Significant behavioral differences in mice aged 1 and 6 months in the BC group were observed. Thus, the time to the first immobile suspension in mice aged 1 month was 39 ± 3 s, while in the same mice at attainment of the age of 6 months, the value of this index was 2.7 times greater (t = 11.3, p < 0.001). Total immobility time decreased 2-fold with age (t = 15.7, p < 0.001); the number of immobile suspensions decreased from 10.8 ± 0.3 times to 7.5 ± 0.3 times (t = 7.8, p < 0.001). The mean time per immobile suspension also decreased from 23.0 ± 1.2 seconds to 15 ± 1.1 seconds (t = 4.9, p < 0.001).
In the false irradiation group (0 Gy), stress caused by manipulations related to irradiation (cell transfer, removal of infant mice from their mothers for up to 5 min, simulation of irradiation at the IGUR-1M facility) in mice at the age of 1 month led to a change in only one indicator — a decrease in the total immobility time by 8% (t = 2.2; p = 0.03) (Table 1). However, such stress led to pronounced changes in 3 out of 4 analyzed parameters (time to the first immobile suspension, total immobility time, number of immobile suspension) when these mice reached the age of 6 months. Thus, in the 0 Gy group mice as compared to the BC group, the time to first static suspension decreased by 16% (t = 2.2, p = 0.03), total immobility time increased by 75% (t = 6.4, p < 0.001), and the number of static suspensions increased by 71% (t = 6.2, p < 0.001). These behavioral changes in mice in the 0 Gy group can be interpreted as a manifestation of a depression-like state. Due to the revealed differences in the BC and 0 Gy groups, the analyzed parameters of the irradiated mice were compared with those of the 0 Gy group of the corresponding age.
In mice at the age of 1 month, no statistically significant differences were found between the 0.1 Gy and 1 Gy groups, or with the 0 Gy group, for all the parameters studied (time to the first immobile suspension, total immobility time, number of immobile suspensions, average time of one immobile suspension). In animals irradiated at a dose of 5 Gy, a lengthening of the time to the first immobile suspension (t = 2.6, p = 0.01), as well as a reduction in total immobility time (t = 4.1, p < 0.001) and mean time per immobile suspension (t = 4.0, p < 0.001) were observed. Such changes can be interpreted as a manifestation of antidepressant-like effect of fractionated irradiation at a total dose of 5.2 Gy.
In mice at the age of 6 months, a statistically significant decrease in the total time of static suspension and the number of static suspensions was detected in all dose groups (Table 2). There was also a statistically significant increase in the time to the first immobile suspension in the 5 Gy group (t = 2.6, p = 0.01) and a decrease in the mean time of one immobile suspension in the 1 Gy group (t = 2.9, p = 0.005). Such changes can be interpreted as manifesting an antidepressant-like effect 5 months after exposure to irradiation at doses of 0.1 Gy and higher.
An important stage in researching the causal relationships between the analyzed factor and its effects is the assessment of dose-effect relationship. The revealed changes depended on the level of radiation exposure. Regression analysis showed that the dose of radiation exposure has a statistically significant effect on the time to the first immobile suspension at the age of 1 month (R2 = 0.03; F = 12.5; p < 0.001) and at the age of 6 months (R2 = 0.02; F = 8.3; p = 0.004) (Fig. 1).
At the same time, the slope coefficients in the equations of dependence of time to the first immobile suspension in mice of different ages did not differ (t = 0.2, p = 0.8), indicating that the effect of fractionated irradiation on the indicator of time to the first immobile suspension did not differ in the same animals at the age of 1 and 6 months. Nevertheless, pronounced age changes are registered in the form of increased time to the first immobile suspension in mice at the age of 6 months compared to one-month-old animals.
Total immobility time was dose-dependent at both 1 month of age (R2 = 0.14; F = 66.6; p << 0.001) and 6 months of age (R2 = 0.13; F = 49.8; p < 0.001) (Figure 2).
Despite the dependence of this index on radiation dose being satisfactorily described by linear functions with close slope angles, this dependence for mice at the age of 6 months (Fig. 2) has a nonlinear character and is better described by a logarithmic function (R2 = 0.19; F = 75.6; p < 0.001), suggesting an age-related change in the reaction of animals to radiation exposure: in mice at the age of 6 months, a decrease in the severity of depressive-like behavior is registered starting from the radiation exposure level of 0.1 Gy, whereas in mice aged 1 month, a statistically significant radiation-induced decrease in depressive-like state is observed only at an irradiation dose of 5 Gy.
The number of immobile suspensions in animals at 1 month of age did not statistically significantly change with increasing radiation dose, whereas in the same mice at 6 months of age, a highly reliable dependence on the level of radiation exposure was observed, which was also best described by a logarithmic function (R2 = 0.18; F = 67.1; p < 0.001) (Fig. 3).
The indicator “number of immobile suspensions clearly shows that irradiated animals react differently to short-term unavoidable stress (suspension by the tail) five months after fractionated irradiation in comparison with the non-irradiated control group: in irradiated one-month-old animals, there were no differences from mice from the false irradiation group, while, in the same mice at the age of 6 months, there was registered a dose-dependent decrease of this indicator starting from the level of 0.1 Gy.
Analysis of the average duration of one immobile suspension also revealed age-related changes in the response to short-term unavoidable stress in mice aged 1 and 6 months: in one-month-old animals, a linear dependence of this index on the radiation dose was revealed (R2 = 0.09; F = 40.5; p < 0.001), while in mice aged 6 months, the average duration of one immobile suspension did not depend on the level of radiation exposure (Fig. 4).
The peculiarities of the long-term experiment to assess the effect of fractionated gamma irradiation on physiological processes associated with the development of a depression-like state are the inevitable influence of such factors as age, sex, stress associated with manipulations during irradiation of animals. While the separate effects of these factors are as given above, in order to obtain a holistic picture of the relationship between these factors, it is necessary to conduct a multivariate analysis. The multivariate variance analysis using the generalized linear model showed that the time to the first immobile suspension was statistically significantly influenced by such factors as sex, age, irradiation dose, while the stress factor had a statistically significant effect only on mice aged 6 months. It was found that the “time to the first immobile suspension” in females was 8 ± 3 s longer than in males (Table 3).
While stress had no statistically significant effect on the time to the first immobile suspension in mice at the age of 1 month, the value of this index significantly decreased in the same animals at 6 months. Radiation exposure had a statistically significant effect on the time to the first static suspension; the value of this index increased by 3 ± 0.8 s with increasing dose by 1 Gy.
According to multivariate variance analysis, the total immobility time depended on age, stress, and radiation dose and was independent of the sex of the mice. The stress factor calculated for animals from the 0 Gy, 0.1 Gy, 1 Gy, and 5 Gy groups caused an average increase of 40 ± 8 s, with the value of this parameter being lower by an average of 49 ± 11 s in mice aged 1 month. Radiation exposure had a statistically significant effect on total immobility time, which decreased by 9.4 ± 1.1 s with increasing dose of 1 Gy (Table 3).
The number of immobile suspensions statistically significantly depended on age, stress, irradiation dose, but did not depend on the sex of animals. Mice aged 6 months showed a decrease in the number of immobile suspensions compared to one-month-old mice. Stress, on average, resulted in an increase in the number of immobile suspensions by 2.9 ± 0.5 in groups exposed to procedures involving irradiated mice. Moreover, stress had an effect on animals at 6 months of age and no effect at 1 month of age. In multivariate variance analysis, such age differences in stress response were expressed as an increase in the coefficient for the stress factor and an equal magnitude decrease in the coefficient for the stress*age factor for mice at 1 month of age, where the stress factor had no effect on the number of immobile suspensions. As the dose increased by 1 Gy, the number of immobile suspensions decreased on average by 0.6 ± 0.1. Such changes were determined mainly by the responses of mice aged 6 months, in which the regression analysis revealed a highly significant dependence of the analyzed index on the irradiation dose (Fig. 3). In mice at the age of 1 month, the number of fixed suspensions according to the regression analysis did not depend on the dose. Such age-related peculiarities are expressed in the decrease of the coefficient for the factor “Irradiation dose” by 0.6 ± 0.1 and compensatory increase of the coefficient for the factor “Age*dose” in mice aged 1 month (Table 2).
The mean time of one static suspension, which statistically significantly depended on age and exposure dose, was independent of sex and stress. In animals aged 6 months, the mean time of one static suspension was 5.7 ± 0.9 s shorter than in animals aged 1 month. Upon irradiation, this index decreased with increasing dose of 1 Gy by an average of 0.14 ± 0.021 s. It should also be noted that a combination of the factors “Sex*age” and “Age*dose” had a statistically significant effect on the value of the average time of one static suspension. In males at the age of 1 month, the time of one static suspension was 3.3 ± 1.1 seconds longer than in the same males at the age of 6 months and females at the age of 1 and 6 months. The influence of modifying effect of age was manifested in the absence of statistically significant dependence of this index on the irradiation dose at the age of 6 months, although at the age of 1 month the mean time of one static suspension decreased with dose by 1.0 ± 0.27 s with increasing dose by 1 Gy (Table 3).
Table 1. Tail suspension test results in 1 month old mice in different experimental groups
|
Group |
Gender |
Time to the first immobile suspension, sec |
Total immobility time, sec |
Number of immobile suspensions (M±SE) |
Average time of one immobile suspension (M±SE), sec |
|
BC |
♂ |
42 ± 5 |
230 ± 7 |
10.9 ± 0.5 |
24 ± 2.1 |
|
♀ |
36 ± 3 |
228 ± 7 |
10.8 ± 0.3 |
22 ± 1.0 |
|
|
♂+♀ |
39 ± 3 |
229 ± 5 |
10.8 ± 0.3 |
23.0 ± 1.2 |
|
|
0 Gy |
♂ |
*28 ± 4 |
226 ± 8 |
11 ± 0.5 |
23.2 ± 1.8 |
|
♀ |
51 ± 10 |
*194 ± 10 |
10.6 ± 0.4 |
*18.3 ± 1.1 |
|
|
+♀ |
39 ± 5 |
*211 ± 7 |
10.8 ± 0.3 |
20.9 ± 1.1 |
|
|
0.1 Gy |
♂ |
†44 ± 3 |
222 ± 7 |
10.9 ± 0.5 |
21.7 ± 1.3 |
|
♀ |
*50 ± 4 |
†222 ± 5 |
10.9 ± 0.4 |
†22.3 ± 1.0 |
|
|
♂+♀ |
47 ± 3 |
226 ± 5 |
10.9 ± 0.3 |
22 ± 0.8 |
|
|
1 Gy |
♂ |
†41 ± 4 |
212 ± 8 |
11.1 ± 0.4 |
20.6 ± 1.2 |
|
♀ |
*54 ± 3 |
†230 ± 6 |
11.4 ± 0.4 |
20.7 ± 1.0 |
|
|
♂+♀ |
*47 ± 2.5 |
217 ± 5 |
11.2 ± 0.3 |
20.6 ± 0.8 |
|
|
5 Gy |
♂ |
†49 ± 4 |
*†181 ± 9 |
11.4 ± 0.5 |
*†16.8 ± 1.1 |
|
♀ |
*64 ± 8 |
*†163 ± 9 |
11.4 ± 0.6 |
*†14.7 ± 1.0 |
|
|
♂+♀ |
*†56 ± 5 |
*†172 ± 7 |
11.4 ± 0.4 |
*†15.7 ± 0.8 |
Table prepared by the authors using their own data
Note: ♂ — males; ♀ — females; * — statistically significant differences from the index in the biological control group, p<0.05; † — statistically significant differences from the index in the 0 Gy group, p<0.05.
Table 2. Tail suspension test results of 6-month-old mice in different experimental groups
|
Groups |
Gender |
Time to the first immobile suspension, sec |
Total immobility time, sec |
Number of immobile suspensions |
Average time of one immobile suspension, sec |
|
BC |
♂ |
‡101 ± 9 |
‡97 ± 8 |
‡7.9 ± 0.5 |
‡12.5 ± 1.0 |
|
♀ |
‡109 ± 6 |
‡114 ± 9 |
‡7.1 ± 0.4 |
‡17.5 ± 1.9 |
|
|
♂+♀ |
‡105 ± 5 |
‡106 ± 6 |
‡7.5 ± 0.3 |
‡15.0 ± 1.1 |
|
|
0 Gy |
♂ |
88 ± 8 |
*168 ± 17 |
*11.7 ± 1.0 |
14.5 ± 1.0 |
|
♀ |
*87 ± 8 |
*204 ± 13 |
*13.9 ± 1.1 |
16.7 ± 1.2 |
|
|
♂+♀ |
*88 ± 6 |
*186 ± 11 |
*12.8 ± 0.8 |
15.6 ± 0.8 |
|
|
0.1 Gy |
♂ |
82 ± 8 |
*133 ± 9 |
*9.7 ± 0.5 |
13.4 ± 0.9 |
|
♀ |
95 ± 5 |
†135 ± 8 |
*†8.7 ± 0.4 |
16.2 ± 1.2 |
|
|
♂+♀ |
*88 ± 5 |
*†134 ± 6 |
*†9.2 ± 0.3 |
14.8 ± 0.8 |
|
|
1 Gy |
♂ |
96 ± 9 |
†106 ± 8 |
†8.9 ± 0.5 |
12.1 ± 0.9 |
|
♀ |
99 ± 8 |
†95 ± 9 |
†7.7 ± 0.4 |
*†12.5 ± 1.3 |
|
|
♂+♀ |
98 ± 6 |
†101 ± 6 |
†8.3 ± 0.3 |
*†12.3 ± 0.8 |
|
|
5 Gy |
♂ |
113 ± 10 |
†104 ± 10 |
†7.2 ± 0.6 |
14.9 ± 1.6 |
|
♀ |
†111 ± 9 |
†103 ± 9 |
†7.9 ± 0.5 |
*†12.9 ± 1.1 |
|
|
♂+♀ |
†112 ± 7 |
†104 ± 7 |
†7.6 ± 0.4 |
13.9 ± 1.0 |
Table prepared by the authors using their own data
Note: ♂ — males; ♀ — females; * — statistically significant differences from the index in the biological control group, p<0.05; † — statistically significant differences from the index in the 0 Gy group, p < 0.05; ‡ — statistically significant differences in the BC groups in mice aged 1 and 6 months, p < 0.05.
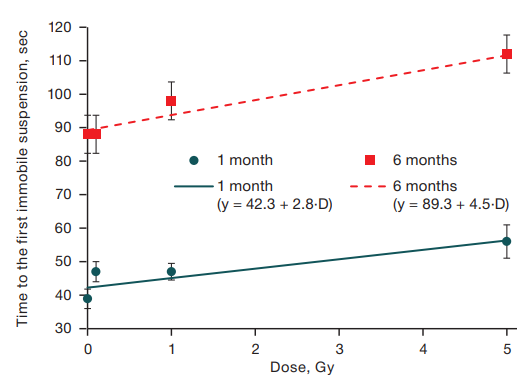
Figure prepared by the authors using their own data
Fig. 1. Dependence of the time to the first immobile suspension on the dose of gamma irradiation in mice aged 1 and 6 months
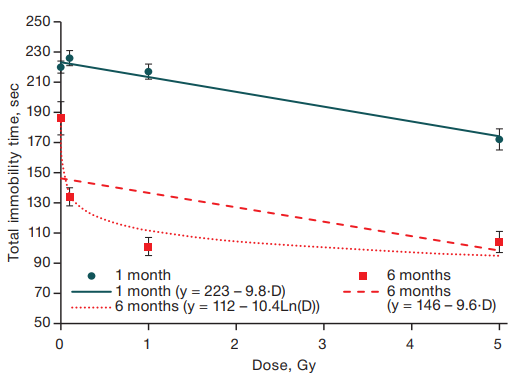
Figure prepared by the authors using their own data
Fig. 2. Dependence of total immobility time on the dose of gamma irradiation in mice aged 1 and 6 months
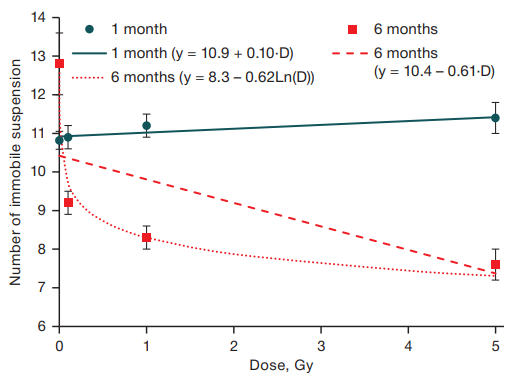
Figure prepared by the authors using their own data
Fig. 3. Dependence of the number of immobile suspensions on the dose of gamma irradiation in mice aged 1 and 6 months
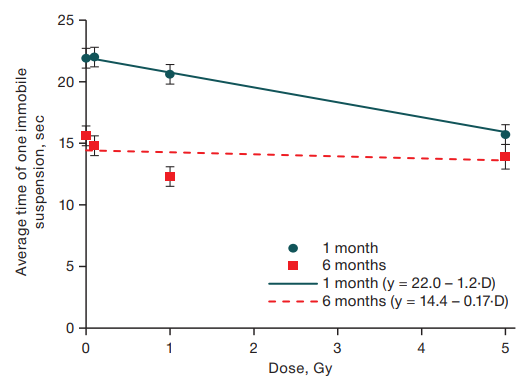
Figure prepared by the authors using their own data
Fig. 4. Dependence of the average time of one immobile suspension on the gamma irradiation dose in mice aged 1 and 6 months
Table 3. Results of assessing the influence of various factors on the indices in the tail-suspension test using multivariate variance analysis in a general linear model
|
Factors |
F |
F |
Coefficients |
|
|
Time to first suspension, sec (R2 = 0.28) |
||||
|
Gender of animals |
6.44 |
0.011 |
male: 8 ± 3 |
female: 0 |
|
Age of animals |
222.23 |
< 0.001 |
1 month: 0 |
6 months: 49 ± 3 |
|
Stress |
1.96 |
0.16 |
stress–: 0 |
stress+: 0 |
|
Radiation dose |
19.85 |
< 0.001 |
D · (3 ± 0.8) |
|
|
Stress*Age |
4.59 |
0.032 |
stress–, 1 month: 0 stress+, 1 month: 0 |
stress–, 6 months: 0 stress+, 6 months: -16 ± 8 |
|
Total suspension time, sec (R2 = 0.356) |
||||
|
Gender of animals |
0.027 |
0.87 |
male: 0 |
female: 0 |
|
Age of animals |
320.9 |
< 0.001 |
1 month: 0 |
6 months: -75 ± 5 |
|
Stress |
7.57 |
0.006 |
stress–: 0 |
stress+: 40 ± 8 |
|
Radiation dose |
73.42 |
< 0.001 |
D · (-9.4 ± 1.1) |
|
|
Stress*age |
19.17 |
< 0.001 |
stress–, 1 month: 0 stress+, 1 month: -49 ± 11 |
stress–, 6 months: 0 stress+, 6 months: 0 |
|
Number of suspensions (R2 = 0.126) |
||||
|
Gender of animals |
0.004 |
0.95 |
male: 0 |
female: 0 |
|
Age of animals |
30.04 |
< 0.001 |
1 month: 0 |
6 months: -0.5 ± 0.04 |
|
Stress |
18.78 |
< 0.001 |
stress–: 0 |
stress+: 2.9 ± 0.5 |
|
Radiation dose |
15.2 |
< 0.001 |
D · (-0.6 ± 0.1) |
|
|
Stress*age |
15.79 |
< 0.001 |
stress–, 1 month: 0 stress+, 1 month: 0 |
stress–, 6 months: 0 stress+, 6 months: -2.8 ± 0.7 |
|
Age*dose |
28.17 |
< 0.001 |
1 months: D · (-0.5 ± 0.04) |
6 months: D 0 |
|
Average time of one suspension, sec (R2 = 0.126) |
||||
|
Gender of animals |
0.002 |
0.97 |
male: 0 |
female: 0 |
|
Age of animals |
114.97 |
< 0.001 |
1 month: 0 |
6 months: -5.7 ± 0.9 |
|
Stress |
1.67 |
0.2 |
stress–: 0 |
stress+: 0 |
|
Radiation dose |
19.42 |
< 0.001 |
D · (-0.14 ± 0.021) |
|
|
Gender*age |
8.57 |
0.004 |
male, 1 month: 3.3 ± 1.1 male, 6 months: 0 |
female, 1 month: 0 female, 6 months: 0 |
|
Age*dose |
13.33 |
< 0.001 |
1 month: D (-1.0 ± 0.27) |
6 months: D · 0 |
Table prepared by the authors using their own data
Note: “stress–” — absence of a stress factor associated with animal irradiation (group BK); “stress+” — presence of a stress factor associated with animal irradiation (groups 0 Gy, 0.1 Gy, 1 Gy, 5 Gy)
DISCUSSION
The study revealed that fractionated irradiation at an early age led to a dose-dependent reduction of depression-like behavior in mice in the tail retention test, maximally expressed at a total dose of 5.2 Gy. This is indicated by changes in all the parameters recorded in this test: the total time of immobility, interpreted as despair behavior, is reduced; animals make longer attempts to free themselves from an unpleasant position before suspension motionless for the first time; the number of such acts of immobile suspension and the average duration of one immobile suspension are reduced.
In animals at the age of 6 months, less pronounced signs of depression-like behavior were observed than at the earlier age: thus, in the biological control group, the total immobility time decreased more than 2-fold with age, while 2.5-fold longer mice tried to actively free themselves before motionless suspension for the first time. At the same time, the previously revealed effects of irradiation were fully preserved: the signs of depression-like behavior of irradiated animals decreased with increasing dose of irradiation.
This effect of irradiation was rather unexpected: the development of depression-like behavior in rodents has been described in the literature at acute irradiation at comparable doses. Thus, when rats at the age of 3 days were irradiated at a dose of 5 Gy, an increase in immobility time in the tail retention test and the Porsolt forced swimming test was observed after 120 days without any change in their general motor activity [12]. In this study, the authors also revealed hypoplasia of the granular layer of the dentate gyrus of the hippocampus, impaired division of neuronal stem cells, and changes in the process of their migration and maturation, which may represent the physiological basis of the behavioral changes [12].
An important role of neurogenetic changes in the dentate gyrus of the hippocampus in the development of postradiation depression was also revealed when studying the effects of targeted irradiation of the dentate gyrus compared to total brain irradiation in mice at the age of 10 days [10]. Here, 3 months after irradiation, an increase in anxiety was detected in the elevated cruciform maze at acute irradiation at doses of 0.25 Gy, along with an increase in anxiety detected in the test of glass bead burying at acute irradiation at doses of 0.5 Gy and an increase in manifestations of depressive behavior in the forced swimming test at acute irradiation at doses of 0.25 Gy. All effects were more pronounced at targeted irradiation of the ventral part of the dentate gyrus.
At fractionated X-ray irradiation in cumulative doses of 0.4 and 0.5 Gy (single dose of 0.1 Gy), more anxious behavior in the open-field test was observed in mice [17]. In long-term modeling of chronic neutron-photon irradiation, an increase in anxiety in the open-field test was revealed in mice at a cumulative dose of 0.4 Gy over 600 days of irradiation [18]. However, a decrease in the severity of the effects with dose fractionation was also shown compared to acute irradiation: while acute irradiation at a dose of 5 Gy resulted in inhibition of neurogenesis in the dentate gyrus, as well as learning and memory deficits in the test for contextual fear conditioning and memory deficits in the test for recognition of new objects, when this dose was divided into 10 daily fractions, no such behavioral changes were observed, and the inhibition of neurogenesis was insignificant [19].
In our studies, fractionated gamma-irradiation during the first month of life at a cumulative dose of 5.2 Gy led not only to a decrease in depression-like behavior, but also, as previously reported, to the formation of the least anxious phenotype: anxiety indices in the elevated cross-shaped maze and the glass bead burial test decreased in animals irradiated at this dose [20]. An increase in anxiety and neophobic behavior was observed at a cumulative dose of 0.1 Gy [20], which, however, was not accompanied by an increase in the manifestations of depression.
It is important to note the revealed influence of early-life stress on the development of signs of depression over long periods following the end of stress exposure. The stress factor in this study is manipulation in the process of irradiation or its imitation over the course of the first month of the animals’ life. Thus, while at the age of 1 month the indices of depression-like behavior of mice in the 0 Gy false irradiation group were close to those of intact animals of the biological control group, at the age of 6 months significant differences were observed indicating the development of depression-like behavior in mice of the false irradiation group. These results are quite consistent with both the results of modeling stress at an early age in mice [21] and clinical data on the increased risk of developing anxiety and depressive psychopathologies in people who have suffered mental traumas in childhood [22][23].
However, according to the obtained data, the indices of depression-like behavior in animals exposed to both stress factor and fractionated irradiation decreased in a dose-dependent manner up to the biological control values at an irradiation dose of 5.2 Gy. Similar results were obtained in a study of diazepam-induced depression in rats, where X-ray irradiation at a dose of 3 Gy divided into 6 fractions led to a decrease in depressive symptomatology and normalization of neurotransmitter levels [24].
A number of studies have also revealed weak neuroprotective effects of a single irradiation at doses up to 0.1 Gy, such as a decrease in signs of proinflammatory activation of microglia, an increase in the density of neurons in the dentate gyrus of the hippocampus, and an increase in the functional activity of mitochondria [1][25][26]. Such changes in the hippocampal dentate gyrus may underlie the described antidepressant symptomatology of irradiated animals.
CONCLUSIONS
- A dose-dependent change in depressive-like behavior in mice under fractionated irradiation in the first month of life (the period of active brain maturation) was revealed; with increasing total dose from 0.1 Gy to 5 Gy, indices of depressive behavior decreased. This pattern is shown both in the early period immediately following the completion of irradiation (at the age of 1 month) and over longer periods of time (at the age of 6 months).
- Stress suffered in early age led to the development of depression-like behavior in adult unexposed mice; however, under simultaneous exposure to stress and fractionated irradiation in doses of 1–5 Gy, the indices of depression-like behavior of mice at the age of 6 months did not differ from intact animals.
References
1. Atamanyuk NI. The effect of moderate and low doses of ionizing radiation of higher nervous activity of human and animals. Extreme medicine. 2023;25(3):5–13 (In Russ.). https://doi.org/10.47183/mes.2023.029
2. Tanguturi SK, Alexander BM. Neurologic complications of radiation therapy. Neurol Clin. 2018; 36(3):599–625. https://doi.org/10.1016/s0733-8619(02)00031-2
3. Pasqual E, Boussin F, Bazyka D, Nordenskjold A, Yamada M, Ozasa K et al. Cognitive effects of low dose of ionizing radiation — Lessons learned and research gaps from epidemiological and biological studies. Environ Int. 2021;147:106295. https://doi.org/10.1016/j.envint.2020.106295
4. Loganovsky K, Marazziti D. Mental health and neuropsychiatric aftermath 35 years after the chernobyl catastrophe: current state and future perspectives. Clin Neuropsychiatry. 2021;18(2):101–6. https://doi.org/10.36131/cnfioritieditore20210204
5. Loganovsky KN, Masiuk SV, Buzunov VA, Marazziti D, Voychulene YS. Radiation risk analysis of neuropsychiatric disorders in ukrainian chornobyl catastrophe liquidators. Front Psychiatry. 2020;(11):553420. https://doi.org/10.3389/fpsyt.2020.553420
6. Contis G, Foley TP, Jr. Depression, suicide ideation, and thyroid tumors among ukrainian adolescents exposed as children to chernobyl radiation. J. Clin. Med. Res. 2015;(7):332–8. https://doi.org/10.14740/jocmr2018w
7. Balashov PP, Bujkov VA, Kolmogorova VV, Burtovaja EJ. Clinic variants of organic disorders with depressive manifestation in exposed to radiation population in the area of radioactive accidents in the South Urals. Siberian herald of psychiatry and addiction psychiatry. 2009;(3):92–5 (In Russ.). EDN: KTYYGB
8. Collett G, Craenen K, Young W, Gilhooly M, Anderson RM. The psychological consequences of (perceived) ionizing radiation exposure: a review on its role in radiation-induced cognitive dysfunction. Int J Radiat Biol. 2020;96(9):1104–18. https://doi.org/10.1080/09553002.2020.1793017
9. Njamnshi AK, Ahidjo N, Ngarka L, Nfor LN, Mengnjo MK, Njamnshi WY, et al. Characterization of the Cognitive and motor changes revealed by the elevated plus maze in an experimental rat model of radiation-induced brain injury. Adv Biomed Res. 2020;9:72. https://doi.org/10.4103/abr.abr_62_20
10. Dos Santos M, Kereselidze D, Gloaguen C, Benadjaoud MA, Tack K, Lestaevel P, Durand C. Development of whole brain versus targeted dentate gyrus irradiation model to explain low to moderate doses of exposure effects in mice. Sci Rep. 2018;8(1):17262. https://doi.org/10.1038/s41598-018-35579-x
11. Whoolery CW, Walker AK, Richardson DR, Lucero MJ, Reynolds RP, Beddow DH, et al. Whole-Body exposure to 28Siradiation dose-dependently disrupts dentate gyrus neurogenesis and proliferation in the short term and new neuron survival and contextual fear conditioning in the long term. Radiat Res. 2017;188(5):532–51. https://doi.org/10.1667/RR14797.1
12. Wang H, Ma Z, Shen H, Wu Z, Liu L, Ren B, et al. Early life irradiation-induced hypoplasia and impairment of neurogenesis in the dentate gyrus and adult depression are mediated by MicroRNA-34a-5p/T-Cell intracytoplasmic antigen-1 pathway. Cells. 2021;10(9):2476. https://doi.org/10.3390/cells10092476
13. Pitzer C, Kurpiers B, Eltokhi A. Sex differences in depression-like behaviors in adult mice depend on endophenotype and strain. Front Behav Neurosci. 2022;16:838122. https://doi.org/10.3389/fnbeh.2022.838122
14. Shishkina EA, Atamanyuk NI, Peretykin AA, Pryakhin EA. Whole organism doses and their uncertainties during mice exposure at the gamma radiobiological installation IGUR-1M. ANRI. 2024;117(2):63–75 (In Russ.). https://doi.org/0.37414/2075-1338-2024-117-2-63-75
15. Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012;59:e3769. https://doi.org/10.3791/3769
16. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2022. Available at: https://www.R-project.org.
17. Koturbash I, Jadavji NM, Kutanzi K, Rodriguez-Juarez R, Kogosov D, Metz GAS, et al. Fractionated low-dose exposure to ionizing radiation leads to DNA damage, epigenetic dysregulation, and behavioral impairment. Environ Epigenet. 2017;2(4):dvw025. https://doi.org/10.1093/eep/dvw025
18. Perez RE, Younger S, Bertheau E, Fallgren CM, Weil MM, Raber J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice. Behav Brain Res. 2020;379:112377. https://doi.org/10.1016/j.bbr.2019.112377
19. Tang FR, Loke WK, Wong P, Khoo BC. Radioprotective effect of ursolic acid in radiation-induced impairment of neurogenesis, learning and memory in adolescent BALB/c mouse. Physiol Behav. 2017;175:37–46. https://doi.org/10.1016/j.physbeh.2017.03.027
20. Atamanyuk NI, Obvintseva NA, Peretykin AA, Pryakhin EA. The dose-dependent effect of fractionated γ-radiation on anxiety-like behavior in neonatal mice. Bull Exp Biol Med. 2024;176(6):727– 30 (In Russ.). https://doi.org/10.1007/s10517-024-06097-w
21. He T, Guo C, Wang C, Hu C, Chen H. Effect of early life stress on anxiety and depressive behaviors in adolescent mice. Brain Behav. 2020;10(3):e01526. https://doi.org/10.1002/brb3.1526
22. Ochi S, Dwivedi Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol Psychiatry. 2023;28(1):141–53. https://doi.org/10.1038/s41380-022-01907-x
23. Juruena MF. Early life stress, depression and epigenetics. Vitam Horm. 2023;(122):307–37. https://doi.org/10.1016/bs.vh.2023.01.004
24. Kaur A, Singla N, Dhawan DK. Low dose X-irradiation mitigates diazepam induced depression in rat brain. Regul Toxicol Pharmacol. 2016;(80):82–90. https://doi.org/10.1016/j.yrtph.2016.06.004
25. Casciati A, Dobos K, Antonelli F, Benedek A, Kempf SJ, Belles M et al. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget. 2016;7(19):28040–58. https://doi.org/10.18632/oncotarget.8575
26. Ung MC, Garrett L, Dalke C, Leitner V, Dragosa D, Hladik D et al. Dose-dependent long-term effects of a single radiation event on behaviour and glial cells. Int J Radiat Biol. 2021;97(2):156–69. https://doi.org/10.1080/09553002.2021.1857455
About the Authors
N. A. ObvintsevaRussian Federation
Chelyabinsk
N. I. Atamanyuk
Russian Federation
Chelyabinsk
I. A. Shaposhnikova
Russian Federation
Chelyabinsk
A. A. Peretykin
Russian Federation
Chelyabinsk
E. A. Pryakhin
Russian Federation
Chelyabinsk
Supplementary files
Review
For citations:
Obvintseva N.A., Atamanyuk N.I., Shaposhnikova I.A., Peretykin A.A., Pryakhin E.A. Assessment of depression-like behavior in mice after fractional gamma irradiation. Extreme Medicine. 2024;26(3):30-39. https://doi.org/10.47183/mes.2024-26-3-30-39

















