Scroll to:
Microparticles as quality criteria for platelet concentrate
https://doi.org/10.47183/mes.2024-26-4-132-140
Abstract
Introduction. Due to the increased requirement for platelet concentrate use in the treatment and prevention of thrombocytopathy, there is a pressing need for the development, improvement and implementation of new approaches to monitoring its quality parameters and safety assessment.
Objective. To conduct a systematic review and analysis of literature data, in order to identify promising approaches to evaluating an adequate analysis of the quality of platelet concentrate to improve the effectiveness and safety of transfusions.
Discussion. The possibilities and advantages of a rational approach to platelet concentrate transfusion are established, while considering the degree of platelet activation required to optimize the preparation of the component. Special attention was paid to methods for evaluating platelet activation. The detection of microparticles based on dynamic light scattering will make it possible to distinguish activated platelets (with a high content of microparticles) from inactive (with a low content of microparticles) platelets during both therapeutic and preventive transfusions and optimize the use of this scarce blood component.
Conclusions. The ability to differentiate platelet concentrates based on the screening of the content of microparticles formed due to activation will contribute to improving the effectiveness and safety of transfusion therapy.
For citations:
Grishina G.V., Kasyanov A.D., Lastochkina D.V., Krobinets I.I., Golovanova I.S., Matvienko O.Yu. Microparticles as quality criteria for platelet concentrate. Extreme Medicine. 2024;26(4):132-140. https://doi.org/10.47183/mes.2024-26-4-132-140
INTRODUCTION
In the last two decades in the Russian Federation, due to the improvement and intensification of treatment programs with the use of PC in many fields of medicine, there has been a steady increase in the need to use platelet concentrates (PC).
Platelets are specialized nuclear-free blood cells which play a key role in stopping bleeding and dangerously blocking healthy blood vessels in thrombosis. Inactivated platelets are flattened spheroids (disks) with a semi-axis ratio 2–8 and a characteristic size of 2–4 µm in diameter. In the activated state, the shape of platelets changes: a part of activated platelets acquires a shape close to spherical. During the transition to the activated state, microparticles with sizes ranging from 50–100 nm are separated from platelets that fall into the intercellular space of blood [1]. An indicator of the proportion of active platelets is the content of microparticles in a given concentrate.
Platelet concentrates are one of the deficit blood components used in hemotransfusions. In order to assess the quality of these concentrates and their proper use, information is needed regarding the proportion of active platelets contained in them [1–4]. This indicator depends on the individual characteristics of the donor, the method of obtaining platelet concentrate from donor blood, as well as the duration and storage conditions of the concentrate. Too large a proportion of microparticles in the concentrate makes it unsuitable for use in transfusion. The possibility of using concentrates with a moderate proportion of active platelets and, consequently, microparticles depends on the characteristics of the patient for whom the concentrate is intended.
In this work, we conduct a systematic review and analysis of published literature to identify promising approaches to assessing adequate platelet concentrate quality assurance to improve the efficiency and safety of transfusions.
DISCUSSION
Properties and functions of platelet microparticles
The formation of microparticles (MPs) or microvesicles is an integral manifestation of cell viability, occurring both in vivo and in vitro. The formation of microparticles is stimulated by pathogens, stress, various damages, and other unfavorable factors [1]. The most active production of microparticles is characteristic of blood formers, as well as endothelial and smooth muscle cells of blood vessels [2]. Among all microparticles in the blood, platelet microparticles (PMs) are the most abundant [3] and account for about 70–90% of the total number of cells. There is a growing scientific and clinical interest in the physiologic role played by platelet microparticles [4]. Compared to platelets, which have an average lifespan of about 10 days, the lifespan of platelet microparticles is measured in minutes [5], while the aspect of removing microparticles from circulation in the bloodstream remains unclear.
Platelet microparticles are 0.1–1 μm fragments released from platelet plasma membranes which undergo activation, stress or apoptosis, with a wide range of biological activities. They have a phospholipid-based (PLP) structure and express functional receptors from platelet membranes. As the most abundant microparticles in the blood, PMs express the procoagulant phosphatidylserine (PS) and likely complement, if not enhance, platelet functions in hemostasis, thrombosis, cancer and inflammation, while also acting as stimulators of tissue regeneration. Their size and structure make PMs indispensable in the system of intercellular interactions with tissue cells as a delivery tool for platelet-carried bioactive molecules, including growth factors, other signaling molecules, and microRNAs.
Considering the reactivity of PMs, they may have different pathophysiologic effects on the cellular environment when interacting with components of the circulatory system. There is also growing evidence that PM production is triggered during donation, component separation, and storage of blood. This may lead to thrombotic and inflammatory side effects in recipients. Evaluation of PMs requires rigorous pre-analytical and analytical procedures, aimed at avoiding the generation of artifacts. At the same it ensures the accurate estimation of the quantity, size redistribution and functional properties of these microparticles [6][7].
In vivo, PMs can be released from platelets under normal physiologic conditions or as a result of activation, stress, or apoptosis. Surface marker studies have shown that PMs are probably the most abundant MPs in healthy individuals, constituting 70–90% of circulating cells in the blood [6], with a range of approximately 100–1000/μL [7]. Megakaryocytes can also release MPs [8], but determining their proportion requires further studies. The remaining MPs are released by endothelial cells, leukocytes, and erythrocytes. Evaluation of PMs still requires the development of rigorous preanalytical and analytical procedures, in order to ensure the most accurate estimation of abundance, size, and functional properties. The number of PMs increases as a result of activation of the coagulation cascade or complement system, as well as by apoptotic signals or shear forces. The number of PMs increases in some prothrombotic and inflammatory diseases, as well as in some cancers [9].
The quantitative content of microparticles in blood is probably related to the state of balance between their formation and utilization [10]. In various pathological conditions, this balance is disturbed, and an increase in the content of platelet microparticles caused by “chronic activation” of platelets is registered in the blood [11]. Platelet microparticles are heterogeneous and characterized by the presence or absence of mitochondria [12], as well as variation in size. Since PMs can express functional receptors from platelet membranes and are PLP-based nanoparticles, they are increasingly considered as tools in the interaction between platelets and tissue cells [13]. Platelet microparticles contain biologically active molecules, including growth factors and other signaling molecules which can transmit messages to neighboring or target cells [14]. Thus, PMs have the potential to influence the cellular environment interacting with the blood network. This is because they expose the procoagulant surface of PLP and can act as a transporter of previously mentioned bioactive molecules, as well as genetic materials including microRNAs [15]. PM generation is also triggered in vitro during blood cell processing and storage [16], potentially causing side effects during transfusion therapy.
Accurate characterization of PMs requires careful preparation of study samples, in order to avoid experimental artifacts. In addition, when evaluating MPs [17], a reasonable choice of analytical methods combining techniques which characterize the cellular origin, number, size, and functional activity of microparticles should be made.
Microparticles as a versatile quality indicator of platelet concentrates
PCs are widely used in clinical practice. In cancer patients, platelets are used to prevent hemorrhagic syndrome. In trauma and surgical patients, PC transfusions are performed to stop bleeding. In addition, the introduction of platelet-rich plasma injections is based on the immunologic functions of platelets.
The quality requirements for platelets intended to prevent and stop bleeding or accelerate wound healing vary widely. The question is whether a single measurable characteristic can describe platelet quality for all applications. A positive answer is provided by Maurer-Spurej, who presents data regarding the use of microparticle measurement in platelet samples as a versatile quality characterization for production, storage, viability assessment, function, and compatibility of PCs [18].
It has been suggested that viable platelets in the platelet concentrate are lost due to storage lesion changes. However, donor variability is not thought to be a major contributing factor [19]. This implies that patients with indications for transfusion of PC containing long-lasting viable platelets in the bloodstream should be transfused as soon as possible after procurement. Traditionally, in vitro platelet quality assessment has been based on these assumptions [20]. In the event of changes in these parameters both during platelet activation and storage, the measurement of CD62 surface receptor expression, ADP-stimulated aggregation level is performed. It has been shown that the release of microparticles by platelets follows activation, and increases during storage of platelet components [21]. Thus, platelet quality is assessed from the perspective of the manufacturer and regulated, in order to ensure consistency and stability of the manufacturing process [22].
Since PC quality assessment is focused on detecting degradation from the beginning to the end of the regulated 5-day shelf-life [23], normative indices have high resolution for small changes in “resting/viable” platelets, while low resolution for “activated/functional” platelets. The characteristics of donor platelet quality are relevant when assessing the immediate response to transfusion. However, maintaining the duration of response depends predominantly on patient characteristics [24][25].
The studies of Maurer-Spurej et al. noted that in 17% of cases (regardless of age) transfusion of donor platelet concentrates did not lead to the expected increase in the number of platelets after transfusion in oncohematologic patients [26]. One possible reason for this unexpectedly high rate of adverse clinical outcomes is that platelet viability is highly dependent on donor characteristics. Indeed, it was found that approximately 33% of donors were found to have pre-activated platelets, as evidenced by high levels of microparticles in the harvested platelet concentrate [27]. Patients with oncopathology usually require platelet transfusion not for reasons of active bleeding, but due to a predisposition to develop bleeding due to low platelet counts secondary to underlying disease and/or therapy. In these patients, donor platelets must remain in the circulatory stream for possible activation. Due to reduced viability, pre-activated platelets are not recommended for storage or use in cancer patients [28]. On the contrary, pre-activated platelets with increased hemostatic activity are believed to be effective in stopping acute bleeding, which is especially important for patients with surgery or trauma [29].
Platelets are used in clinical practice for a wide variety of purposes. For this reason, the quality of the platelet concentrate should match the specific transfusion objective. Consequently, the ideal quality parameter for predicting platelet function after PC transfusion should be to distinguish between resting/viable and pre-activated/highly functional platelets with the same resolution across the entire viability/functionality spectrum [28][29].
The indicator of microparticle content as fragmentation and heterogeneity of platelets can be considered as a quality criterion for platelet production, storage, viability, function, and compatibility [30][31]. The heterogeneity of platelet concentrates, considering the degree of activation by microparticles, enables the process of procurement to be corrected, and PC to be differentiated for prophylactic and therapeutic transfusions.
If platelets are more viable (i.e., capable of surviving certain storage conditions, transportation, irradiation, and other influences), then they are, by definition, less functional. This fact has been known since the 1970s, when researchers tried to determine the optimal storage temperature for platelet concentrates. Platelets stored at room temperature were found to be more viable, while refrigerated platelets showed better functional activity. This is based on an assessment of the criterion of changes in hemostatic parameters in volunteers receiving aspirin or in patients with thrombocytopenia [32]. Maintenance of platelet viability is an important role of anticoagulants. Concentrates rich in viable platelets are homogeneous in composition, containing predominantly disc-shaped platelets and few (or no) microparticles or microaggregates [27]. In contrast, platelets which have undergone long-term storage and refrigeration, or otherwise activated, are expected to be heterogeneous. They are expected to contain platelets with a high level of polydispersity due to polymorphism, high surface expression of activation markers, large numbers of microparticles, and the presence of microaggregates [33].
The heterogeneity of platelet concentrates increases during storage [34] as well as during pathogenreduction [23] and varies greatly in healthy donors [35]. The greatest contribution to platelet heterogeneity is their microparticle content.
At the present time, two important questions require consideration: firstly, whether microparticles in blood components have a potentially pathogenic effect; and secondly, how the process of preparation, additional processing and storage of blood components affect the release of microparticles. Recently, the importance of microparticles as indicators of PC quality has been heightened due to their potential physiological and pathophysiological roles. The importance of microparticles in transfusion medicine is recognized since microparticles are present in both plasma and cellular blood products [7]. The pathophysiological effects of PMs related to the regulation of immune responses have raised obvious concerns about the potential deleterious effects associated with blood transfusion in immunocompromised patients [36][37]. There is strong evidence that both blood cell-derived microparticles and platelet microparticles are generated during the production and storage of all blood components, plasma for transfusion, and red blood cell and platelet concentrates prepared from whole blood by apheresis [38][39]. PM release is induced by stimuli such as shear stress resulting from activation or apoptosis of cells during storage, as well as their contact with the walls of the storage container [38]. Cellular expression by-products are formed [40], when platelet concentrates are stored at from +20 to –24°C or erythrocyte units at from +1 to –6°C.
Platelet microparticles appear to be present in higher amounts in PC prepared by apheresis, rather than in the platelet concentrate obtained from the leukotrombotic layer. The specifics of the apheresis procedure protocol or the type of cell separator used have been shown to potentially influence the extent of PM release during processing [41]. Platelet microparticles do not appear to be removed by leukoreduction [42]. On the other hand, leukofiltration of whole blood has been found to reduce the risk of subsequent PM formation [43]. Fresh frozen plasma prepared after overnight exposure of whole blood at +4°C contained more PMs than that obtained 8 h after blood collection [44]. At the same time, according to George JN et al., Cryoprecipitate containing highly concentrated PMs had a potentially more pronounced hemostatic effect when compared to the initial plasma [45].
In order to ensure transfusion therapy safety, a better understanding of the PM release mechanism during processing and storage of blood components through careful sample preparation and a combination of analytical techniques [46] is important. Suspected complications of transfusion include: increased risk of infectious complications; as well as renal, respiratory and multi-organ failure [37]. Some authors (Khorana AA, Francis CW et al.) have found a correlation between the increased incidence of venous thrombosis and embolism after administration of platelet concentrates with the presence of PMs [47], known to exhibit higher procoagulant activity (50–100-fold), when compared to equivalent activated platelets [48]. Although PM half-life is thought to be very short due to phagocytosis by macrophages, recent experiments have shown that it can be approximately 5.8 h when platelet concentrates are administered to patients with severe thrombocytopenia [49].
Phosphatidylserine-expressing PMs can activate innate immune cells. This can lead to an inflammatory response mediating transfusion-related acute lung injury (TRALI) [50]. Platelet microparticles may also contribute to immunosuppressive effects occurring during transfusion, indirectly explaining the occurrence of post-transfusion infections or cancer recurrences [40]. Another possible MP side effect is the potential high risk of alloimmunization against blood cell antigens, since they may have a high immunological potential [51].
Platelet refractoriness is a situation in which a patient fails to have the expected clinically significant response to a PC transfusion [52]: a complication seen in 27% of platelet recipients [53]. Refractoriness to platelet transfusions is defined as two consecutive platelet transfusions resulting in an insufficient increase in corrected count increments (CCI). The threshold below which the CCI is considered insufficient depends on the time of measurement. A CCI of less than 5000–7500 platelets/μL measured in a recipient blood sample taken 1 h after transfusion characterizes poor recovery, while a CCI of less than 5000 platelets/μL in a sample taken 24 hours after transfusion characterizes poor survival. Patients with immune refractoriness have a low post-transfusion CCI both 1 and 24 hours after transfusion, which can be eliminated (or not) by selection of appropriate HLA/HPA platelet concentrates [54]. However, an increase in platelet count at 1 h, followed by a significant decrease in platelet count 24 h after transfusion is detectable even in the absence of documented alloimmunization. It has been suggested that inadequate platelet quality is one of the reasons why cancer patients may have particularly poor platelet survival in the period of 24 h after transfusion [12]. In addition to complicating the treatment process itself, the cost of bed days more than doubles when treating patients with platelet resistance, when compared with patients without refractoriness with hospital stays 21 days longer [55].
Microparticles are markers of prothrombotic inflammation. Patients who become refractory to platelet transfusion often have concomitant fever or systemic inflammation, detectable as increased microparticles [56]. Consequently, homogeneous platelets may be a better choice for cancer patients at risk of developing refractoriness, whereas heterogeneous platelets may lead to the development of complications. Thus, transfusion of platelets from microparticle-rich donors may be the most optimal option. In all likelihood, transfusion of heterogeneous platelets to patients whose immune systems are hyperactivated may push them to a tipping point where they become immune to platelet transfusion. In independent studies by Cortés-Puch et al, and Flegel et al, found that not transfusing heterogeneous platelets prophylactically may prevent refractoriness. A similar concept was analyzed in an experimental study on animals in which the combination of bacterial infection and transfusion of “older” erythrocytes containing high concentrations of microparticles led to an increased risk of mortality [57][58].
Heterogeneous platelet concentrates contain pre-activated platelets capable of reacting quickly when they enter the bloodstream. Thus, heterogeneous platelets have a more pronounced functional activity and have been shown to stop bleeding faster than homogeneous viable platelets [33].
The introduction of microparticle measurements for the quality and safety control of platelet concentrates could eliminate the influence of pathogen inactivation. It could also contribute to supplement solutions and 7-day storage conditions for PC. Inventory management based on this indicator could lead to optimization of the treatment process and significantly reduce costs.
Evaluation of platelet microparticle content
A number of experimental methods have been used to detect and analyze microparticles of platelet origin. These include: flow cytometry; nanoparticle trajectory analysis; electron microscopy; atomic force microscopy, and dynamic light scattering [59–61].
The most informative of these methods is flow cytometry. This method measures elastic light scattering intensity from a single cell or particle, sequentially passing through the laser beam focusing zone. It subsequently processes this data using special mathematical algorithms. In this case, the characteristics of frontal and lateral light scattering provide an idea of the size and structure of the cell. It also considers the level of fluorescence of chemical compounds included in the cell. This method requires the use of rather complex and expensive equipment. It also requires a large amount of time to be spent on preparation and analysis, so it is of little use for rapid screening of platelet concentrates.
Dynamic light scattering (DLS) was proposed as an express method for the rapid control of microparticle content in platelet concentrates. It is a rather effective method for measuring the size and size distribution of particles in a liquid. In DLS analyzers, the time dependence of the intensity of laser radiation scattered by particles suspended in the liquid is directly measured. Based on this dependence, the distribution of particles in the coordinates “particle diameter — relative intensity of radiation” scattered by particles of a given diameter (intensity distribution) is reconstructed. Two areas can be distinguished in such distributions: in the interval from 0.05 to 0.5 µm, corresponding to microparticles; and in the area from 1 to several µm, corresponding to platelets. This principle is the basis for the evaluation of platelet concentrates using a specialized DLS analyzer ThromboLUX produced by the Canadian company LightIntegra Technology [59].
When using the ThromboLUX technique with the tested platelet concentrate, three measurements are performed: at 37°C; cooling to 20°C; and reheating to 37°C. The study by Maurer-Spurej E. et al. demonstrated the result of measurements of particle distributions by mean of the DLS method [27]. It was found that upon cooling from 37°C to 20°C, the proportion of platelets decreased from 72 to 65%, while microparticles increased from 26 to 31%. Reheating to 37°C restored the former ratio of microparticles and platelets.
The ThromboLux method has been shown to correlate well with flow cytometry and electron microscopy data [59]. The content of microparticles can be a fairly versatile indicator used to assess the quality of platelets in the concentrate. In an experimental study [18], a scheme for selecting the optimal platelet concentrate for different categories of patients was shown based on the analysis of a large number of data. In particular, the ThromboLux technique has been used in the USA and Canada for the comparative analysis of the quality of PCs obtained from different donors or by different methods from the blood of one donor [60].
Microparticles in platelet concentrates have recently been studied using a versatile DLS analyzer, the Malvern Zetasizer [61]. This instrument collects scattered radiation at an angle of 173° (backscattering technology), enabling measurements to be performed for low-transparent samples. Particle distributions measured for undiluted platelet concentrates are the most informative. Figure 1 shows the scattered intensity distributions of microparticles and platelets measured under the above conditions. Three measurements were made for each of the samples studied. The first measurement was made at a temperature of 37°C, characterizing the initial state of the concentrate. The second measurement characterizes the resistance of platelets to temperature stress, i.e. activation when the temperature is lowered to 20°C. The third measurement was performed after increasing the temperature from 20 to 37°C, its results allowed the ability of platelets to recover from stress to be assessed.
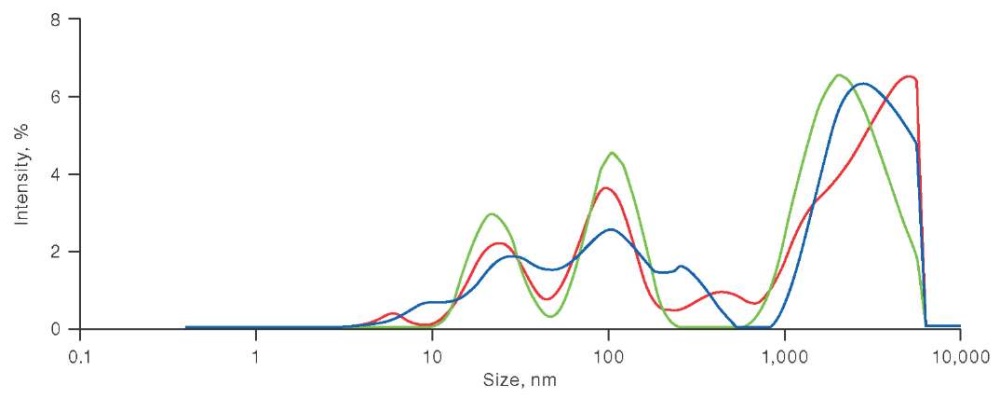
The figure is based on the authors’ use of data from references [59]
Fig. 1. Scattered intensity distribution of microparticles and platelets in platelet concentrate
Note: red color — at a temperature of 37°C (initial state); green color — at a temperature of 20°C; blue color — after increasing the temperature from 20 to 37°C.
The data presented in Figure 1 shows a large proportion of microparticles in PC at 20°C (green line), and its decrease when heated to 37°C (blue line). Labrie A. et al. analyzed platelet concentrates obtained by different methods: by apheresis; from platelet-enriched plasma; and from leukocyte-phosphorus film. The measured size distributions of microparticles clearly show peaks corresponding to exosomes (smaller particles with a diameter not exceeding 100 μm) and microparticles of platelet origin [59]. However, depending on the method of PC production, the positions of these peaks on the scale of particle diameters differ markedly [60].
In recent years, technologies for the preparation, processing, storage, and use of PCs have developed intensively. They now require new methods for assessing the quality and safety of blood components which meet modern standards [59–63]. The method of microparticle detection in PCs as one of the possibilities to preserve their quality characteristics and safety during subsequent transfusions is shown in Fig. 2.
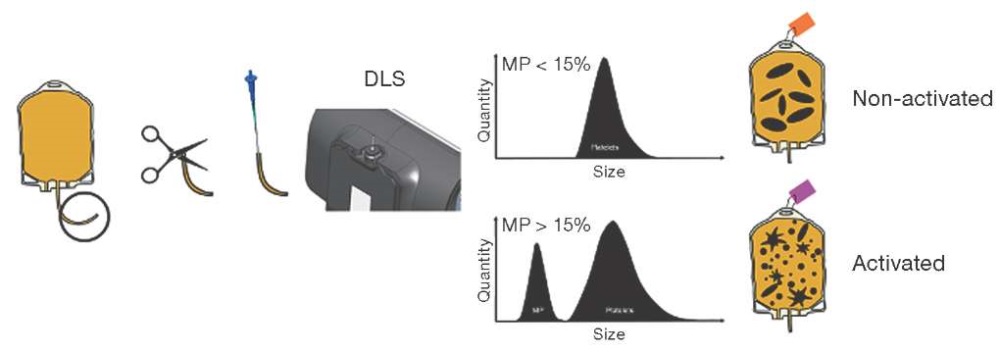
Figure prepared based on data from [60]
Fig. 2. A method for detecting the content of microparticles which permits differentiation between activated and non-activated platelet concentrates [60]
Figure 2 shows the steps required to routinely manage platelet supply in a blood bank: a sample is obtained from the platelet concentrate; the sample is loaded into a capillary for DLS measurement; a DLS test is performed to identify microparticles; and the reported microparticle content then used to identify activated platelets. It takes the average user 3 min 23 s to prepare the DLS system for the test, obtain and test the sample according to the protocol, and then label the platelet bag. The focus of this protocol is to determine the composition of particles present in platelet transfusions and to use microparticles as biomarkers of platelet activation. Platelet transfusions are labeled as inactivated or activated based on a threshold of 15% microparticle percentage. Towards the end of the shelf life, an increase in the number of circulating platelet-derived MPs is noted in the PC. This indicates excessive platelet activation and/or apoptosis and loss of platelet functional activity, ultimately leading to a decrease in the expected therapeutic effect of the PC. Determination of the number of platelet-derived MPs by DLS method may be a promising method for assessing the quality of PC.
A promising development is the use of a domestic technique involving dynamic light scattering. This technique analyzes the content of microparticles in platelet concentrates as one of the criteria for quality control of blood components. Modern analysis will perform routine screening of microparticles in platelet-rich plasma or platelet concentrates, with validation of the results, leading to the formation of a conclusion on the possibility of using PC for subsequent transfusion.
CONCLUSION
With the development of modern technologies, the search for new methods to determine the quality and safety of blood components, including platelet concentrates, is becoming increasingly important. For routine screening of PC, a rapid and non-invasive test can be performed. Its purpose is to evaluate the characteristics of those platelets which are important for the recipient. The parameter to be investigated could be the determination of microparticle content. By measuring the composition of the platelet concentrate, the component’s storage characteristics and resistance to additional stress can be determined, while its optimal use can be justified.
The method presented herein to evaluate the quality and safety of platelet concentrates is based on dynamic light scattering, thus offering several advantages over previous tests. The introduction of the method into clinical practice will enable the effectiveness of PC use before transfusion to be assessed. This is important because multiple transfusions of PC in patients may cause adverse events. Patients who experience blood loss due to trauma or surgery should be transfused with more active platelets containing high levels of microparticles. When transfusing PC to cancer patients, activated platelets are undesirable. In this case, it is recommended that a platelet concentrate with a minimum content of microparticles be used. However, the use of this method in clinical practice requires further study.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows: Galina V. Grishina — writing the manuscript, data analysis, article design, approval of the final version of the manuscript for publication; Andrei D. Kasyanov — planning and development of research design, article design; Daria V. Lastochkina, Irina S. Golovanova — test analysis; Olesya Yu. Motvienko — data analysis; Irina I. Krobinets — development of research design, approval of the final version of the manuscript for publication.
References
1. Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7(2):105–15. https://doi.org/10.4161/org.7.2.15782
2. Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23. https://doi.org/10.1016/j.thromres.2008.06.006
3. Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34(6):392–401. https://doi.org/10.1111/j.1365-2362.2004.01355.x
4. Burnouf T, Goubran HA, Chou ML, Devos D, Radosevic M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28(4):155–66. https://doi.org/10.1016/j.blre.2014.04.002
5. Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis. 2006;36(2):182–7. https://doi.org/10.1016/j.bcmd.2005.12.019
6. Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137(1):36–48. https://doi.org/10.1111/j.1365-2141.2007.06514.x
7. Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20(1):1–26. https://doi.org/10.1016/j.tmrv.2005.08.001
8. Siljander PR. Platelet-derived microparticles — an updated perspective. Thromb Res. 2011;127 Suppl 2:S30-3. https://doi.org/10.1016/S0049-3848(10)70152-3
9. Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21(3):157–71. https://doi.org/10.1016/j.blre.2006.09.001
10. Ayers L, Nieuwland R, Kohler M, Kraenkel N, Ferry B, Leeson P. Dynamic microvesicle release and clearance within the cardiovascular system: triggers and mechanisms. Clin Sci (Lond). 2015;129(11):915–31. https://doi.org/10.1042/CS20140623
11. Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost. 2005;94(3):488–92. https://doi.org/10.1160/TH05-03-0201
12. Marcoux G, Duchez AC, Rousseau M, Lévesque T, Boudreau LH, Thibault L et al. Microparticle and mitochondrial release during extended storage of different types of platelet concentrates. Platelets. 2017;28(3):272–80. https://doi.org/10.1080/09537104.2016.1218455
13. Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30(5):450–9. https://doi.org/10.1016/s0301-472x(02)00791-9
14. Delabranche X, Berger A, Boisramé-Helms J, Meziani F. Microparticles and infectious diseases. Med Mal Infect. 2012;42(8):335–43. https://doi.org/10.1016/j.medmal.2012.05.011
15. Laffont B, Corduan A, Plé H, Duchez AC, Cloutier N, Boilard E et al. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122(2):253–61. https://doi.org/10.1182/blood-2013-03-492801
16. Saas P, Angelot F, Bardiaux L, Seilles E, Garnache-Ottou F, Perruche S. Phosphatidylserine-expressing cell by-products in transfusion: A pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol. 2012;19(3):90–7. https://doi.org/10.1016/j.tracli.2012.02.002
17. Momen-Heravi F, Balaj L, Alian S, Tigges J, Toxavidis V, Ericsson M et al. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. https://doi.org/10.3389/fphys.2012.00354
18. Maurer-Spurej E, Chipperfield K. Could Microparticles Be the Universal Quality Indicator for Platelet Viability and Function? J Blood Transfus. 2016;2016:6140239. https://doi.org/10.1155/2016/6140239
19. Mittal K, Kaur R. Platelet storage lesion: An update. Asian J Transfus Sci. 2015;9(1):1–3. https://doi.org/10.4103/0973-6247.150933
20. Holme S. Storage and quality assessment of platelets. Vox Sang. 1998;74(2):207–16. https://doi.org/10.1111/j.1423-0410.1998.tb05422.x
21. Pienimaeki-Roemer A, Kuhlmann K, Böttcher A, Konovalova T, Black A, Orsó E et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion. 2015;55(3):507–21. https://doi.org/10.1111/trf.12874
22. Farrugia A, Vamvakas E. Toward a patient-based paradigm for blood transfusion. J Blood Med. 2014;5:5–13. https://doi.org/10.2147/JBM.S55769
23. Johnson L, Schubert P, Tan S, Devine DV, Marks DC. Extended storage and glucose exhaustion are associated with apoptotic changes in platelets stored in additive solution. Transfusion. 2016;56(2):360–8. https://doi.org/10.1111/trf.13345
24. Murphy S. Utility of in vitro tests in predicting the in vivo viability of stored PLTs. Transfusion. 2004;44(4):618–9. https://doi.org/10.1111/j.1537-2995.2004.00355.x
25. Apelseth TO, Bruserud O, Wentzel-Larsen T, Hervig T. Therapeutic efficacy of platelet transfusion in patients with acute leukemia: an evaluation of methods. Transfusion. 2010;50(4):766–75. https://doi.org/10.1111/j.1537-2995.2009.02540.x
26. Maurer-Spurej E, Labrie A, Pittendreigh C, Chipperfield K, Smith C, Heddle N et al. Platelet quality measured with dynamic light scattering correlates with transfusion outcome in hematologic malignancies. Transfusion. 2009;49(11):2276–84. https://doi.org/10.1111/j.1537-2995.2009.02302.x
27. Maurer-Spurej E, Larsen R, Labrie A, Heaton A, Chipperfield K. Microparticle content of platelet concentrates is predicted by donor microparticles and is altered by production methods and stress. Transfus Apher Sci. 2016;55(1):35–43. https://doi.org/10.1016/j.transci.2016.07.010
28. Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M et al. American Society of Clinical Oncology. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1519–38. https://doi.org/10.1200/JCO.2001.19.5.1519
29. Getz TM, Montgomery RK, Bynum JA, Aden JK, Pidcoke HF, Cap AP. Storage of platelets at 4°C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56(6):1320–8. https://doi.org/10.1111/trf.13511
30. Johnson L, Reade MC, Hyland RA, Tan S, Marks DC. In vitro comparison of cryopreserved and liquid platelets: potential clinical implications. Transfusion. 2015;55(4):83–47. https://doi.org/10.1111/trf.12915
31. Johnson L, Tan S, Wood B, Davis A, Marks DC. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion. 2016;56(7):1807–18. https://doi.org/10.1111/trf.13630
32. Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion. 1976;16(1):20–3. https://doi.org/10.1046/j.1537-2995.1976.16176130832.x
33. Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK et al. Hemostatic function of apheresis platelets stored at 4°C and 22°C. Shock. 2014;41 Suppl 1(01):54–61. https://doi.org/10.1097/SHK.0000000000000082
34. Johnson L, Schubert P, Tan S, Devine DV, Marks DC. Extended storage and glucose exhaustion are associated with apoptotic changes in platelets stored in additive solution. Transfusion. 2016;56(2):360–8. https://doi.org/10.1111/trf.13345
35. Phang M, Lincz L, Seldon M, Garg ML. Acute supplementation with eicosapentaenoic acid reduces platelet microparticle activity in healthy subjects. J Nutr Biochem. 2012;23(9):1128–33. https://doi.org/10.1016/j.jnutbio.2011.06.006
36. Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, Kim HK, Ryu KW, Bae JM, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39(2):184–91. https://doi.org/10.1016/s0959-8049(02)00596-8
37. Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cellderived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions? Blood Transfus. 2012;10 Suppl 2:s25–38. https://doi.org/10.2450/2012.006S
38. Bode AP, Orton SM, Frye MJ, Udis BJ. Vesiculation of platelets during in vitro aging. Blood. 1991;77(4):887–95.
39. Krailadsiri P, Seghatchian J. Are all leucodepleted platelet concentrates equivalent? Comparison of Cobe LRS Turbo, Haemonetics MCS+ LD, and filtered pooled buffy-coat-derived platelets. Vox Sang. 2000;78(3):171–5. https://doi.org/10.1159/000031176
40. Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21(6):327–48. https://doi.org/10.1016/j.blre.2007.07.003
41. Rank A, Nieuwland R, Liebhardt S, Iberer M, Grützner S, Toth B, et al. Apheresis platelet concentrates contain plateletderived and endothelial cell-derived microparticles. Vox Sang. 2011;100(2):179–86. https://doi.org/10.1111/j.1423-0410.2010.01385.x
42. Devine DV, Bradley AJ, Maurer E, Levin E, Chahal S, Serrano K, et al. Effects of prestorage white cell reduction on platelet aggregate formation and the activation state of platelets and plasma enzyme systems. Transfusion. 1999;39(7):724–34. https://doi.org/10.1046/j.1537-2995.1999.39070724.x
43. Sugawara A, Nollet KE, Yajima K, Saito S, Ohto H. Preventing platelet-derived microparticle formation--and possible side effects-with prestorage leukofiltration of whole blood. Arch Pathol Lab Med. 2010;134(5):771–5. https://doi.org/10.5858/134.5.771
44. Lawrie AS, Cardigan RA, Williamson LM, Machin SJ, Mackie IJ. The dynamics of clot formation in fresh-frozen plasma. Vox Sang. 2008;94(4):306–44. https://doi.org/10.1111/j.1423-0410.2008.01037.x
45. George JN, Pickett EB, Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood. 1986;68(1):307–9.
46. Strasser EF, Happ S, Weiss DR, Pfeiffer A, Zimmermann R, Eckstein R. Microparticle detection in platelet products by three different methods. Transfusion. 2013;53(1):156–66. https://doi.org/10.1111/j.1537-2995.2012.03720.x
47. Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377–81. https://doi.org/10.1001/archinte.168.21.2377
48. Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–34.
49. Rank A, Nieuwland R, Crispin A, Grützner S, Iberer M, Toth B et al. Clearance of platelet microparticles in vivo. Platelets. 2011;22(2):111–6. https://doi.org/10.3109/09537104.2010.520373
50. Saas P, Angelot F, Bardiaux L, Seilles E, Garnache-Ottou F, Perruche S. Phosphatidylserine-expressing cell by-products in transfusion: A pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol. 2012;19(3):90–7. https://doi.org/10.1016/j.tracli.2012.02.002
51. Kitazawa J, Nollet K, Morioka H, Tanaka K, Inomata M, Kubuki Y et al. Non-D Rh antibodies appearing after apheresis platelet transfusion: stimulation by red cells or microparticles? Vox Sang. 2011;100(4):395–400. https://doi.org/10.1111/j.1423-0410.2010.01435.x
52. Rebulla P. A mini-review on platelet refractoriness. Haematologica. 2005;90(2):24753.
53. Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105(10):4106–14. https://doi.org/10.1182/blood-2003-08-2724
54. Hess JR, Trachtenberg FL, Assmann SF, Triulzi DJ, Kaufman RM, Strauss RG, et al. Clinical and laboratory correlates of platelet alloimmunization and refractoriness in the PLADO trial. Vox Sang. 2016;111(3):281–91. https://doi.org/10.1111/vox.12411
55. Meehan KR, Matias CO, Rathore SS, Sandler SG, Kallich J, LaBrecque J, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol. 2000;64(4):251–6. https://doi.org/10.1002/1096-8652(200008)64:43.0.co;2-n.C.O
56. Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–3. https://doi.org/10.1126/science.1181928
57. Cortés-Puch I, Remy KE, Solomon SB, Sun J, Wang D, Al-Hamad M, et al. In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells markedly alters outcome. Transfusion. 2015;55(11):2564–75. https://doi.org/.1111/trf.13275
58. Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol. 2014;165(1):3–16. https://doi.org/10.1111/bjh.12747
59. Labrie A, Marshall A, Bedi H, Maurer-Spurej E. Characterization of platelet concentrates using dynamic light scattering. Transfus Med Hemother. 2013;40(2):93–100. https://doi.org/10.1159/000350362
60. Millar D, Hayes C, Jones J, Klapper E, Kniep JN, Luu HS, et al. Comparison of the platelet activation status of single-donor platelets obtained with two different cell separator technologies. Transfusion. 2020;60(9):2067–78. https://doi.org/10.1111/trf.15934
61. Li M, Zhao Y, Chen X, Du X, Luo Y, Li Y, et al. Comparative analysis of the quality of platelet concentrates produced by apheresis procedures, platelet rich plasma, and buffy coat. Transfusion. 2024;64(2):367–79. https://doi.org/10.1111/trf.17704
62. Kishchenko VV, Sirotkina OV, Sidorkevich SV, Vavilova TV. Platelet vesicles are a potential marker of platelet concentrate quality. Preventive and clinical medicine. 2020;77(4):93–101 (In Russ.). https://doi.org/10.47843/2074-9120_2020_4_93
63. Ponomarenko EA, Ignatova AA, Fedorova DV, Zharkov PA, Panteleev MA. Functional activity of platelets: physiology and laboratory diagnostic methods. Issues of hematology/oncology and immunopathology in pediatrics. 2019;18(3):112–19 (In Russ.). https://doi.org/10.24287/1726-1708-2019-18-3-112-119
About the Authors
G. V. GrishinaRussian Federation
St. Petersburg
A. D. Kasyanov
Russian Federation
St. Petersburg
D. V. Lastochkina
Russian Federation
St. Petersburg
I. I. Krobinets
Russian Federation
St. Petersburg
I. S. Golovanova
Russian Federation
St. Petersburg
O. Yu. Matvienko
Russian Federation
St. Petersburg
Review
For citations:
Grishina G.V., Kasyanov A.D., Lastochkina D.V., Krobinets I.I., Golovanova I.S., Matvienko O.Yu. Microparticles as quality criteria for platelet concentrate. Extreme Medicine. 2024;26(4):132-140. https://doi.org/10.47183/mes.2024-26-4-132-140
















