Scroll to:
Substance P and stress are associated with the development of chronic urticaria
https://doi.org/10.47183/mes.2024-26-4-21-26
Abstract
Introduction. Allergic diseases are a pressing challenge in practical healthcare, attracting increased attention of various medical specialists. The pathogenesis of stress-induced urticaria is driven by neurogenic immune inflammation, accompanied by an increase in the level of neuropeptide substance P (SP).
Objective. Assessment of the relationship between stress factors and substance P levels with the purpose of justifying the use of SP as a biomarker for assessing the clinical course and prognosis of the disease in patients with chronic urticaria.
Materials and methods. The study was involved 165 adults aged 18–68 years. The main group included 97 patients with the confirmed diagnosis of chronic urticaria (CU) who were treated in a hospital setting in the period from 2018 to 2023. The comparison group included 68 practically healthy individuals, comparable in gender and age with the study group of patients. The level of substance P in the blood serum was estimated by immunoenzymatic techniques (Infinite F50 Tecan, Austria), using a CEA393Hu test system. Statistical processing of the results was performed using the STATA 18 software package (StataCorp LLC).
Results. An increase in the production of substance P to 220.62 pg/mL in CU patients, compared to 96.57 pg/mL in the reference group (p < 0.001), was observed. The logistic regression revealed an association between stress and substance P levels in CU patients. Thus, an increase in the concentration of substance P by 1 pg/mL led to a 1.02-fold increase in the CU risk. The CU risk increased by 3 times in the presence of a stress situation as a trigger.
Conclusions. The constructed multivariant logistic regression model produced positive values of the model parameters (p ≤ 0.01). This indicates the correlation between the increased blood levels of substance P under the impact of stress factors and the risk of chronic urticaria development. The data obtained suggests that the concentration of substance P in the blood of CU patients can be considered as a potential diagnostic biomarker. This biomarker can be recommended for extending panel screening tests to clarify the pathogenesis of the disease, thus improving the differential diagnosis of the disease and facilitating early detection of patients with stress-induced urticaria.
For citations:
Mikryukova N.V., Kalinina N.M. Substance P and stress are associated with the development of chronic urticaria. Extreme Medicine. 2024;26(4):21-26. https://doi.org/10.47183/mes.2024-26-4-21-26
INTRODUCTION
Allergic diseases as an urgent problem of practical healthcare have been attracting increased attention of physicians of various specialties. At present, the interrelation of immunologic and neurogenic links is considered as the leading mechanism in the formation of inflammatory response in allergic diseases.
Urticaria is a serious problem in modern allergology, ranking third after allergic rhinitis and bronchial asthma in terms of incidence [1]. Stress is a umbrella term denoting a number of physiological reactions of the organism in response to adverse environmental factors [2]. Such a response encompasses three interrelated concepts: stimuli (both internal and external) that cause stress; physiological and behavioral reactions activated in response to these stimuli; and pathological consequences of excessive stimulation. Psychogenic factors act as a link in the sequence of immunologic events and lead to disease exacerbation, being in close connection with the main factors of pathogenesis [3].
At present, immunologists adhere to the concept of neurogenic inflammation as the main link in the formation and exacerbation of chronic urticaria (CU), which is caused by excessive release of hypothalamic neuropeptides conjugated to skin neuropeptides produced in keratinocytes, endothelial cells, etc. Most researchers agree that mental factors may potentiate the course of allergic diseases. Some authors also emphasize the possibility of urticaria aggravation by psychoemotional stress under the influence of negative emotions [4]. In this case, activation of cortical areas due to stress leads to changes in the production of substance P (SP) by the adrenal glands and descending autonomic fibers [5].
Recent studies confirm the importance of stress in the development of CU [6][7]. A high incidence of post-traumatic stress disorders under the action of anxiety among patients with this nosology was established [8][9]. A significant proportion of CU patients have a history of stressful situations long before the onset of symptoms and signs of this disease [10]. Psychogenic factors stimulate the hypothalamus-pituitary axis, associated with activation of autonomic nervous system centers and release of neurotransmitters (neuropeptides and hormones) affecting the effector systems of the body (immune, cardiovascular, and skin) [5][11]. Chronic stressors lead to an increase in the density of cutaneous nerve fibers, increased production of mast cells, nerve growth factor (NGF), and certain neuropeptides [12]. Wang et al. [13] demonstrated the activation of mast cells (MCs) of both brain and skin under exposure to stress factors in animal experiments.
Mast cells express a variety of receptors that allow them to recognize and respond to a wide range of infectious pathogens and endogenous molecules produced by damaged tissues. For example, the high-affinity immunoglobulin E receptor (FcεRI) and the tyrosine kinase receptor (KIT) are expressed on MCs, which play an important role in the development of allergic reactions and in the immune response during worm invasion [14]. At the same time, mast cells express microbial pattern recognition receptors, i.e., the Toll-like receptor (TLR) and the NOD-like receptor [14]. MCs participate in Th2-type immune inflammation through alarmin receptors of the interleukin family, such as for IL-33 and receptor for thymic stromal lymphopoietin (TSLP) [15]. Mas-related G-protein coupled receptor X2 (MRGPRX2) recognizes neuropeptides, antimicrobial peptides, and insect venom peptides. In addition, these receptors are also present on other effector cells of the immune system, such as basophils and eosinophils [16].
Membrane protein of the adhesion G protein-coupled receptor E2 (ADGRE2) group is a mechanical sensor in mast cells. It induces degranulation in response to vibration exposure in people suffering from vibration urticaria, being a signal of helminth penetration and migration through the skin [17]. Thus, mast cells play an important role in human defense against helminths, bacteria, as well as animal and insect venoms. The release of corticotropin-releasing factor from eosinophils and sensory neurons (under stress) leads to MC degranulation via corticotropin-releasing hormone receptors [12].
The development or relapse of urticaria is frequently caused by stress factors. The leading link in its pathogenesis is neurogenic immune inflammation, accompanied by an increase in the level of neuropeptide substance P (SP) [12]. SP is a neuropeptide of the peripheral endings of sensitive C fibers of the skin, having a wide range of direct and indirect biological effects leading to pathophysiologic responses such as edema, vasodilation, and pruritus [17]. SP is expressed in the central and peripheral nervous system and immune cells. It is a neuropeptide whose biological activity is manifested through two receptors on the cell membrane — neurokinin G-protein coupled receptor (NKR) and MRGPRX2. It is known that MCs in patients with chronic urticaria express MRGPRX2 in greater amounts [18], and the neurotransmitter SP is the most informative diagnostic marker in patients with chronic urticaria [19]. NKR activation mediates neural signaling pathways associated with the occurrence of sensations and various emotional responses.
SP plays an important role in the feeling of pain due to its function of transmitting information about tissue damage from peripheral receptors to the central nervous system, where it is converted into pain sensations. While pain signals are transmitted along the axons of the somatosensory region of the brain, sensory neurons also release SP in the area of damaged tissue [20]. This subsequently leads to mast cell degranulation, vascular dilation due to relaxation of vascular smooth muscle, and chemotaxis of immune system cells. This interaction between the immune and nervous systems is referred to as neurogenic inflammation [21]. In addition, SP was shown to increase the expression of endothelial and leukocyte adhesion molecules on microvascular endothelial cells, leading to leukocyte diapedesis [22]. There are examples of synergistic action of various mast cell triggers. Thus, Tarakanova et al. showed that SP increases the expression of IL-33 ST2 receptor, and IL-33 increases the expression of NKR on human mast cells [23]. This leads to an increase in the secretion of IL-1β, which is a key proinflammatory cytokine.
Despite the achieved progress, the mechanism of neurogenic inflammation in CU remains to be elucidated. This link of pathogenesis continues to be underestimated in the modern clinical protocols for the management of CU patients.
In this research, we set out to analyze the relationship between stress factors and substance P levels in order to justify the possibility of using SP as a biomarker for assessing the clinical course and prognosis of the disease in CU patients.
МАТERIALS AND METHODS
The study was carried out using the facilities of the Nikiforov Russian Center of Emergency and Radiation Medicine. The study involved 165 adults aged 18–68 years, with 89 men and 76 women. The main group included 97 patients (45 men and 52 women) with a confirmed diagnosis of chronic urticaria, treated in a hospital setting in the 2018–2023 period. The comparison group consisted of 68 practically healthy individuals, comparable in gender and age with the main group, without urticaria symptoms and other allergic/somatic pathology [24].
The criterion for inclusion of patients in the main group was the presence of recurrent urticarial rashes and/or angioedema for more than six weeks. The diagnosis of chronic urticaria was established in accordance with the Federal Clinical Guidelines for the Diagnosis and Treatment of Urticaria [25].
In the course of the study, the patients’ medical and life history was collected. The questions that formed the basis for the formation of a group of risk factors for the development or exacerbation of chronic urticaria were clarified during the interview. Special attention was paid to stress factors as potential triggers for the onset or exacerbation of CU.
The serum level of SP was determined in patients with chronic urticaria and in the comparison group by enzyme immunoassay (Infinite F50 Tecan, Austria) using a CEA393Hu test system (Cloud-Clone Corp., China). Blood samples for serum preparation were collected into a vacutainer with a clotting activator and centrifuged at 3000 rpm for 10 min. Serum samples were stored in Eppendorf type tubes at –80 °C. All kit components and serum samples were kept at room temperature +22 °C prior to testing.
Statistical processing of data was performed using the STATA 18 software package (StataCorp LLC). Data samples were compared using the Mann–Whitney U-criterion. The critical level of significance was taken as p ≤ 0.05.
RESULTS AND DISCUSSION
An examination of the CU patients revealed all of them to be in the stage of disease exacerbation at the time of the study. Both study groups were comparable in terms of descriptive characteristics (gender and age).
In the course of the study, statistically significant differences in SP serum levels of patients with CU were obtained. Thus, the SP level in CU patients was more than 56% higher than in the comparison group. The descriptive characteristics of the patients and SP neuropeptide concentrations are presented in Table 1.
Table 1. Descriptive characteristics and SP levels in patients with chronic urticaria and in the comparison group
Indicator/characteristic | Comparison group, n = 68 | Patients with chronic urticaria, n = 97 |
Age, years | 41.10±12.52 | 38.59±8.96 |
Gender m/f | 44/24 | 45/52 |
Substance P, pg/mL | 96.57 [ 78.04–138.16] | 220.62 [ 127.30–302.65] * |
Table prepared by the authors using their own data
Note: data are presented as Me [Q1–Q3].
* — statistical significance of the differences between the main and comparison groups, p < 0.05.
In order to independently assess biomarkers and the probability of developing chronic urticaria, a multivariate regression model with direct sequential inclusion of statistically significant variables of prognostic factors (SP serum concentrations, presence of a stressful situation, age, and gender) was constructed. The characterization of multiple logistic regression is presented in Table 2. The positive values of model parameters (with a significance level of p ≤ 0.01) indicate that exposure to a stressor and an increased concentration of SP in the blood are associated with an increased risk of chronic urticaria. The influence of other factors was not significant.
Table 2. Multivariate logistic regression model including age, gender, trigger stress, substance P level
Chronic urticaria | Odds ratio | p-value | [ 95% confidence interval] |
Stress | 2.873 | 0.014 | 0.15–0.81 |
Substance Р | 1.015 | 0.000 | 1.01–1.02 |
Gender | 0.611 | 0.210 | 1.01–1.02 |
Age | 1.0309 | 0.100 | 0.99–1.07 |
Table prepared by the authors using their own data
According to the regression analysis data presented in Table 2, a 1 pg/mL increase in the concentration of SP increased the chance of CU by 1.02 times. In the presence of a stressful situation as a trigger, the risk of CU increased by 2.873 times. The data on the increase of SP levels in patients with urticaria relapse as a result of a stressful situation indicated the possibility of using the quantitative level of neurotransmitter SP as a biomarker to identify a trigger of CU.
In order to determine the effectiveness and quality of the model for calculating the risk of CU, a ROC curve (Receiver Operator Characteristic) was constructed and the AUC (area under the curve) was calculated. The corresponding data are presented in Fig. 1.
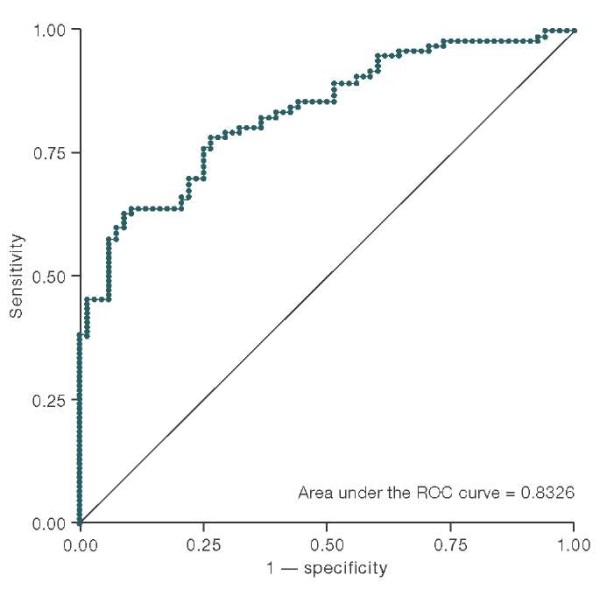
Figure prepared by the authors using their own data
Fig. 1. ROC curve constructed to evaluate the association model of stress, substance P, gender, and age with chronic urticaria
The multivariate logistic regression model can be used to predict the probability of a relationship between the development of chronic urticaria and exposure to risk factors (stress, gender, age, increased concentration of SP). In the logistic regression model, the cut-off point allows us to suggest the association or absence of the disease. Thus, if the predicted probability exceeds the cut-off threshold, the disease is present; otherwise we conclude that it is absent. The quality of correct diagnosis of the presence or absence of the disease is assessed by the sensitivity of the model Se (the proportion of correctly predicted cases of the disease) and specificity Sp (the proportion of correctly predicted cases of the disease absence).
The overall sensitivity of the model was evaluated using the ROC curve showing the dependence of sensitivity on the value of 1 — specificity. The overall sensitivity of the model was defined as the area of the AUC figure under the ROC curve (its value was 0.8326). This value exceeds 0.8, which allows us to conclude that the model has a high prognostic power. The optimal cutoff threshold was determined as a balance point between sensitivity and specificity of the model. The most optimal was the cutoff criterion of 0.7, since it showed the most optimal ratio of specificity and sensitivity of the risk model of CU development depending on prognostic risk factors.
The greatest value for the diagnosis of urticaria was found for stress factors and SP concentrations. The data on the increase of SP concentration in patients with urticaria exacerbation as a result of a stressful situation indicate the possibility of using the neurotransmitter SP as a biomarker to identify the trigger of CU. The presence of clinical symptoms and elevation of SP in the bloodstream indicate the disease, which makes it possible to adjust therapy to achieve a more sustainable effect.
SP is a proinflammatory stress-related neuropeptide released from sensory nerve endings, being a key mediator in CNS-skin communication. It causes mast cell degranulation and the appearance of blisters and/or angioedema in CU. SP is one of the key neurotransmitters that is the first to respond to a stressor, thus contributing to the ongoing inflammation in the skin with a neurogenic component [19].
The increase in the level of SP in patients suffering from CU in relation to the comparison group was statistically significant, which is consistent with the data of other studies [26][19][27][28]. Some studies [29][30] investigated the relationship between the level of SP and the severity of depression in patients with CU. This data confirmed the causal relationship between the development of CU and depression. However, the identified relationships require further in-depth research.
In our study, according to the developed statistical model, elevated blood levels of SP and stress exposure were predictive risk factors for the development of chronic urticaria. Stress-induced CU may accompany various somatic diseases, aggravating their course. An analysis of SP dynamics in other pathologies may improve the current understanding of the relationship between stress and the disease in terms of immune interactions, thus forming the basis for targeted interventions and improved approaches to disease diagnosis and treatment.
It is important to assess the impact of stress in patients with CU for their timely referral to a psychotherapist for psychological or psychopharmacological support to reduce the severity of urticaria manifestations.
Conclusion
Thus, the study revealed a statistically significant increase in the serum neuropeptide substance P in patients with CU. By constructing a multivariate logistic regression model, positive values of the model parameters (with a significance level of p ≤ 0.01) were obtained, indicating that it is the effect of the stress factor and an increase in the substance P blood level that is associated with an increase in the patients with chronic urticaria. The constructed logistic model had a high overall sensitivity, which allows us to conclude about its high predictive power. Based on the data obtained, the substance P blood level in patients with CU can be considered as a potential diagnostic biomarker that can be recommended for expanding the panel of screening tests clarifying the CU pathogenesis, which will improve the differential diagnosis and ensure early detection of patients with stress-induced urticaria for the pathogenetic therapy.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows. Natalya V. Mikryukova — literature analysis, writing a text; Natalia M. Kalinina — editing, making fundamental changes, final approval of the article version.
References
1. Kolkhir PV. Development of the endotypic classification of chronic spontaneous urticaria based on the study of a complex of biomarkers with a personalized approach to therapy: abst. dis. Doc. Sci.(Med.). M., 2016 (In Russ.). EDN: ZPXVPZ
2. Selye H. A syndrome produced by diverse nocuous agents. Nature.1936;138(3479):32. https://doi.org/10.1176/jnp.10.2.230a
3. McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci.2004;1032:1–7. https://doi.org/10.1196/annals.1314.001
4. Staubach P, Dechene M, Metz M, Magerl M, Siebenhaar F, Weller K, et al. High Prevalence of Mental Disorders and Emotional Distress in Patients with Chronic Spontaneous Urticaria. ActaDermVenereol. 2011;91(5):557–61 https://doi.org/10.2340/00015555-1109
5. Pondeljak N, Lugović-Mihić L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. ClinTher. 2020;42(5):757–70. https://doi.org/10.1016/j.clinthera.2020.03.008
6. Lindsay K, Goulding J, Solomon M, Broom B. Treating chronic spontaneous urticaria using a brief ‘whole person’ treatment approach: a proof-of-concept study. Clin Transl Allergy. 2015;5:40. https://doi.org/10.1186/s13601-015-0082-7
7. Schut C, Magerl M, Hawro T, et al. Disease activity and stress are linked in a subpopulation of chronic spontaneous urticaria patients. Allergy. 2020;75(1):224–6. https://doi.org/10.1111/all.14015
8. Donnelly J, Ridge K, O’Donovan R, Conlon N, Dunne PJ. Psychosocial factors and chronic spontaneous urticaria: a systematic review. BMC Psychol.2023;11(1):239. https://doi.org/10.1186/s40359-023-01284-2
9. Gupta MA, Gupta AK. Chronic idiopathic urticaria and post-traumatic stress disorder (PTSD): an under-recognized comorbidity. Clin Dermatol. 2012;30(3):351–4. https://doi.org/10.1016/j.clindermatol.2012.01.012
10. Ridge K, Conlon N, Hennessy M, Dunne PJ. Feasibility assessment of an 8-week attention-based training programme in the management of chronic spontaneous urticarial. Pilot and Feasibility Studies.2021;7(1):103 https://doi.org/10.1186/s40814-021-00841-z
11. Deussing JM, Chen A. The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol Rev. 2018;98(4):2225–86. https://doi.org/doi:10.1152/physrev.00042.2017
12. Keller JJ. Cutaneous neuropeptides: the missing link between psychological stress and chronic inflammatory skin disease?. ArchDermatolRes. 2023;315(7):1875–81. https://doi.org/10.1007/s00403-023-02542-4
13. Wang F, Yang TB, Kim BS. The Return of the Mast Cell: New Roles in Neuroimmune Itch Biology. J InvestDermatol. 2020;140(5):945–51. https://doi.org/10.1016/j.jid.2019.12.011
14. Haidl ID, Marshall JS. Human mast cell activation with viruses and pathogen products. Methods Mol Biol.2015;1220:179–201. https://doi.org/10.1007/978-1-4939-1568-2_12
15. Tsuzuki H, Arinobu Y, Miyawaki K, et al. Functional interleukin-33 receptors are expressed in early progenitor stages of allergyrelated granulocytes. Immunology.2017;150(1):64–73. https://doi.org/10.1111/imm.12667
16. Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy. 2020; 75(9): 2229–42. https://doi.org/10.1111/all.14213
17. Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018;142:381–93. https://doi.org/10.1016/j.jaci.2018.01.034
18. Fujisawa D, Kashiwakura J, Kita H, et al. Expression of Masrelated gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy ClinImmunol. 2014; 134(3): 622–33. https://doi.org/10.1016/j.jaci.2014.05.004
19. Basak PY, Erturan I, Yuksel O, Kazanoglu OO, Vural H. Evaluation of serum neuropeptide levels in patients with chronic urticarial. Indian J DermatolVenereolLeprol. 2014;80(5):483. https://doi.org/10.4103/0378-6323.140345
20. Gouin O, L’Herondelle K, Lebonvallet N, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–61. https://doi.org/10.1007/s13238-017-0395-5
21. Choi JE, Di NardoА. Skin neurogenic inflammation. SeminImmunopathol. 2018;40(3):249–59. https://doi.org/10.1007/s00281-018-0675-z
22. Takashima A. Harnessing DCs by substance P. Blood. 2013;121(15):2815–16. https://doi.org/10.1182/blood-2013-02-483354
23. Taracanova A, Tsilioni I, Conti P, Norwitz ER, Leeman SE, Theoharides TC. Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. ProcNatlAcadSciUSA. 2018;115(40):9381–90. https://doi.org/10.1073/pnas.1810133115
24. Mikryukova NV, Kalinina NM Immunopathogenesis of chronic urticaria in adults (scientific review). Preventive and clinical medicine. 2020; 3(76):77–85 (In Russ.). EDN: DTDBOG
25. Federal clinical guidelines. Urticaria. Danilycheva IV, Il’ina NI, Luss LV, et al. Russian Allergological Journal. 2018;15 (5):47–62 (In Russ.).
26. Metz M, Krull C, Hawro T, Saluja R, Groffik A, Stanger C, et al. Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J Invest Dermatol. 2014;134:2833–36. https://doi.org/10.1038/jid.2014.226
27. Zheng W, Wang J, Zhu W, Xu C, He S. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: induction of histamine release and basophil accumulation by substance P. Cell BiolToxicol. 2016;32:217–28. https://doi.org/10.1007/s10565-016-9330-4
28. Fadaee J, Khoshkhui M, Emadzadeh M, Hashemy SI, FaridHosseini R, JabbariAzad F, et al. Evaluation of Serum Substance P Level in Chronic Urticaria and Correlation with Disease Severity. Iran J Allergy Asthma Immunol. 2020;19(1):18–26. https://doi.org/10.18502/ijaai.v19i1.2414
29. Tedeschi A, Lorini M, Asero R. No evidence of increased serum substance P levels in chronic urticaria patients with and without demonstrable circulating vasoactive factors. Clin Exp Dermatol. 2005;30(2):171–5. https://doi.org/10.1111/j.1365-2230.2005.01732.x
30. Memet B, Vurgun E, Barlas F, Metz M, Maurer M, Kocatürk E. In Chronic Spontaneous Urticaria, Comorbid Depression Linked to Higher Disease Activity, and Substance P Levels. Front Psychiatry. 2021;26(12):667978. https://doi.org/10.3389/fpsyt.2021.667978
About the Authors
N. V. MikryukovaRussian Federation
St. Petersburg
N. M. Kalinina
Russian Federation
St. Petersburg
Review
For citations:
Mikryukova N.V., Kalinina N.M. Substance P and stress are associated with the development of chronic urticaria. Extreme Medicine. 2024;26(4):21-26. https://doi.org/10.47183/mes.2024-26-4-21-26

















