Scroll to:
Hybrid imaging techniques in the assessment of epileptic foci: A clinical case
https://doi.org/10.47183/mes.2025-240
Abstract
Introduction. Localization of an epileptic focus (EF) in patients with pharmacoresistant focal epilepsy can be a challenging task, and the detection of multiple EFs limits the possibilities of surgical treatment. A more accurate description of the structure and metabolism of EFs may provide additional information for managing such patients.
Clinical case description. A 35-year-old patient suffered from pharmacoresistant focal epilepsy, which debuted at the age of 24 with grand mal seizures followed by focal episodes with fading and tonic tension of the left arm. The MRI revealed bilateral mesial temporal sclerosis with more pronounced changes on the left; however, the EEG revealed epileptiform activity in the right temporal region, which created diagnostic difficulties in determining the primary epileptogenic zone. To clarify the location of the lesion, a comprehensive examination was performed, including PET/MRI, which revealed pronounced hypometabolism in the right temporal lobe (with a difference of up to 31% compared with the contralateral side) while maintaining symmetrical accumulation of RPh in the hippocampus. Additionally, diffusion kurtosis imaging (DKI) was performed, which showed a significant decrease in radial and median kurtosis, as well as axonal water fraction in the right temporal lobe (up to 1.33 SD from normal). Invasive EEG monitoring confirmed the bilateral nature of epileptogenicity with a predominance of right-hemisphere seizure initiation.
Conclusions. The combined use of PET/MRI and DKI increases the accuracy of detection of epileptogenic foci in pharmacoresistant epilepsy. Hybrid imaging allows for a comprehensive assessment of metabolic and microstructural changes, which is important for planning surgical treatment. Data integration helps differentiate primary and secondary foci, thus optimizing patient management tactics. The introduction of automated analysis for standardization of diagnostics is promising.
Keywords
For citations:
Nadelyaev R.V., Dolgushin M.B., Rostovtseva T.M., Baranova E.A., Voronkova Yu.A., Gavrilova E.Yu., Rubleva Yu.V. Hybrid imaging techniques in the assessment of epileptic foci: A clinical case. Extreme Medicine. 2025;27(2):176-182. https://doi.org/10.47183/mes.2025-240
INTRODUCTION
Structural focal epilepsy shows pharmacoresistance in 30–40% of cases [1]. This course of the disease forces specialists to resort to surgical methods of treatment [2]. The modern concept describing the pathophysiological basis of structural focal epilepsy implies the presence of an epileptogenic zone (EZ) in the brain substance, which, in turn, includes not only the focus of morphological changes, but also areas of functional changes [16][17]. In this regard, complete resection of the epileptogenic zone is one of the most effective surgical methods to achieve a long-term seizure-free period [3]. Up to 57% of patients who undergo resection of a suspected epileptic focus (EF) become completely seizure-free (according to Engel’s classification — Ia) [4, 5], and 87% of patients cease to have severe epileptic seizures (according to Engel’s classification — Ib) [5]. In this regard, it is critically important to identify the epileptogenic zone and determine the resection boundaries at the stage of preoperative planning.
The conventional set of diagnostic methods, which involves analysis of the clinical picture, noninvasive scalp electroencephalography (EEG), and magnetic resonance imaging (MRI) of the brain according to the epileptic protocol, does not always allow lateralization and localization of the EF. In turn, invasive EEG monitoring with the installation of depth brain electrodes, although exhibiting high sensitivity and being the gold standard in localization and assessment of the prevalence of EF [6], is associated with certain difficulties and risks of various complications [7]. The current research directions in studying epilepsy are aimed at a continued search for more accurate and safe diagnostic methods. In this connection, the presented clinical case demonstrates the potential of combining hybrid imaging techniques of PET/MRI with 18F-fluorodeoxyglucose (FDG) and a promising method of diffusion kurtosis imaging (DKI) [8] in assessing epileptogenic foci in a patient with structural focal epilepsy.
CLINICAL CASE DESCRIPTION
Patient A., 35 years old, was admitted to the neurological department of the Federal Center of Brain Research and Neurotechnologies with complaints of absence seizures, sometimes in combination with tonic tension of the left arm, followed by amnesia, as well as grand mal epilepsy. The onset of the disease at the age of 24 began with a grand mal seizure that developed during sleep, accompanied by wheezing, cyanosis, and post-seizure confusion. The patient turned to a neurologist only one year later, after a second seizure attack. According to the results of the examination, epilepsy of unspecified etiology with bilateral tonic-clonic seizures was diagnosed. An MRI scan of the brain revealed bihemispheric cortical atrophy, suggesting a possible sclerotic change in the right hippocampus. EEG analysis recorded an interictal epileptiform activity in the right frontotemporal region. The combination therapy with antiepileptic drugs resolved generalized seizures (grand mal epilepsy); however, focal nonmotor seizures of the absence type appeared with an average rate of once per month and persisted despite repeated changes in the treatment regimen. Therefore, the patient was admitted to the Federal Center of Brain Research and Neurotechnologies to undergo an in-depth examination in order to localize the epileptogenic zone and consider the possibility of surgical treatment of epilepsy.
The patient underwent MRI of the brain according to the epileptic protocol (isotropic T1 and T2 FLAIR pulse sequence 1×1×1 mm, T2 pulse sequence in the hippocampus plane, T2 and T2 FLAIR pulse sequence perpendicular to the hippocampus plane, T2 pulse sequence in the plane of the anterior and posterior commissures) and daily video EEG monitoring. The analysis of MR images revealed a decrease in the volume of both hippocampuses with a disorder of their structure, which was regarded as bilateral mesial temporal sclerosis, with the changes being noticeably more pronounced in the left hippocampal-amygdalar complex (Fig. 1). No other structural changes characteristic of the potential epileptogenic zone were found.
According to the MRI data shown in Fig. 1, the patient had a bilateral decrease in hippocampal volume with signs of their structural changes in the form of a hyperintensive signal, which were convincing signs of bilateral mesial temporal sclerosis. Daily video EEG monitoring recorded epileptiform activity, represented by acute-slow wave complexes in the right temporal region, occasionally extending to the right frontal-central region. No epileptic seizures were observed.
Given the incomplete correspondence of EEG and MRI data, which made it difficult to lateralize the potential primary epileptogenic zone, it was decided to perform PET/MRI of the brain with 18F-FDG using an integrated SIGNA PET/MR system (GE Healthcare, USA) (Fig. 2). A visual and semi-quantitative analysis of PET/MRI images was performed with the calculation of the standardized uptake volume (SUV) of radiopharmaceutical (RPh) in the areas of interest and in the contralateral areas. The study established a pronounced hyperfixation of the RPh by the substance of the anterior sections of the right temporal lobe in comparison with the contralateral side and a difference of up to 31%. The accumulation of 18F-FDG in the hippocampus and amygdala remained symmetrical.
The data presented in Fig. 2 shows a marked decrease in the 18F-FDG accumulation in the right temporal lobe, which indicates hypometabolic activity in the substance of the right temporal lobe.
In addition to brain PET/MRI, the patient underwent diffusion kurtosis imaging (DKI) as part of the same study. DKI was performed on the same SIGNA PET/MR tomograph with a 3T magnetic field using a 24-channel head coil according to the protocol with three b-factors (0, 1000, 2500) and with gradients in 60 directions, an isotropic voxel size of 3×3×3 mm and a matrix of 80×80 voxels. Postprocessing was performed in the Explore DTI and SPM12 software (MATLAB R2023a). It included correction of the movement of 4D diffusion volumes, correction of Gibbs outliers and Gaussian smoothing. In total, 10 parametric maps were obtained, which were subsequently combined with the anatomical T1 FSPGR series. The areas of interest (both temporal lobes) were semi-automatically segmented based on an individual anatomical atlas, pre-generated using FastSurferCNN using T1 FSPGR series (Desikan–Killiany atlas) [19]. The maps of the mean (MK) and radial (RK) kurtosis, as well as the map of the axonal water fraction (AWF), presented particular interest (Fig. 3). These DKI parameters in the right temporal lobe demonstrated the greatest decrease compared to the relatively small-size norm database previously recruited from healthy volunteers (n = 15) in another study [8]. A decrease of more than one standard deviation (up to 1.33 SD) from the average value according to the norm base was recorded. In addition, there was a visual and quantitative decrease in MK, RK, and AWF values in the substance of the anterior sections of the right temporal lobe relative to the contralateral side.
The maps of radial (Fig. 3a), median (Fig. 3b) kurtosis, and axonal water fraction (Fig. 3b) demonstrate a visual asymmetry of signal intensity between the temporal lobes (D < S), which is confirmed by quantitative comparison with the norm database (the largest decrease by 1.33 SD was observed in the radial kurtosis map).
In order to confirm or refute the assumption of the presence of the primary epileptogenic zone in the right temporal lobe, it was decided to conduct invasive EEG monitoring. Four intracranial electrodes were installed: one in the hippocampus and the corpus amygdaloideum on both sides; monitoring was carried out for nine days. According to the results of the study, a high-index interictal epileptiform activity was recorded under all electrodes with its greater representation on the right, ictal activity in the form of electrographic seizures, also more often with initiation on the right. During electrical stimulation, focal autonomic seizures were recorded with the same frequency when both the left and right corpus amygdaloideum were stimulated (Fig. 4, 5).
Figure 4 shows an electrographic attack with an initiation zone in the left hippocampus: low-amplitude fast-wave activity in the area of the left hippocampus (electrode No. 4, contacts 1–2) extending to 2–4 contacts of the hippocampus and to the corpus amygdaloideum (electrode No. 95 contacts 1–3) with evolution in frequency and amplitude.
Figure 5 shows an electrographic attack with an initiation zone in the right hippocampus: rhythmic fast-wave activity in the area of the right hippocampus (electrode No. 64, contacts 2–3) extending to 1–2 contacts of the hippocampus and to the corpus amygdaloideum (electrode No. 4, 2–3 contacts → evolution of ictal activity in frequency and amplitude).
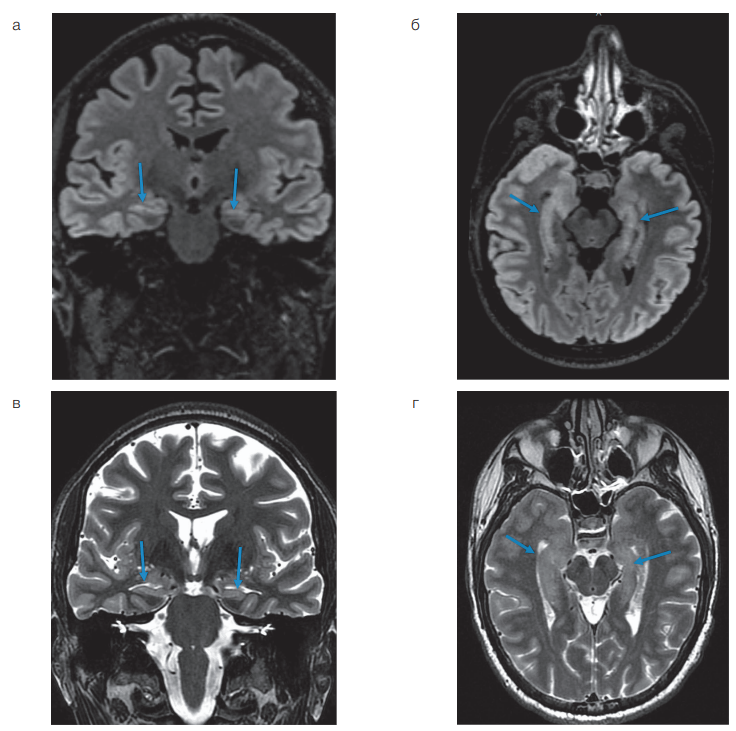
Figure prepared by the authors based on their own data
Fig. 1. MRI of the brain according to the epileptic protocol in a patient with bilateral mesial temporal sclerosis
Note: a — Т2 FLAIR image in the coronal plane, aligned along the long axis of the hippocampus; b — Т2 FLAIR image in the axial plane aligned along the long axis of the hippocampus; c — Т2-weighted image in the coronal plane aligned along the long axis of the hippocampus; d — Т2-weighted image in an axial plane aligned along the long axis of the hippocampus. The arrows indicate the hippocampus.
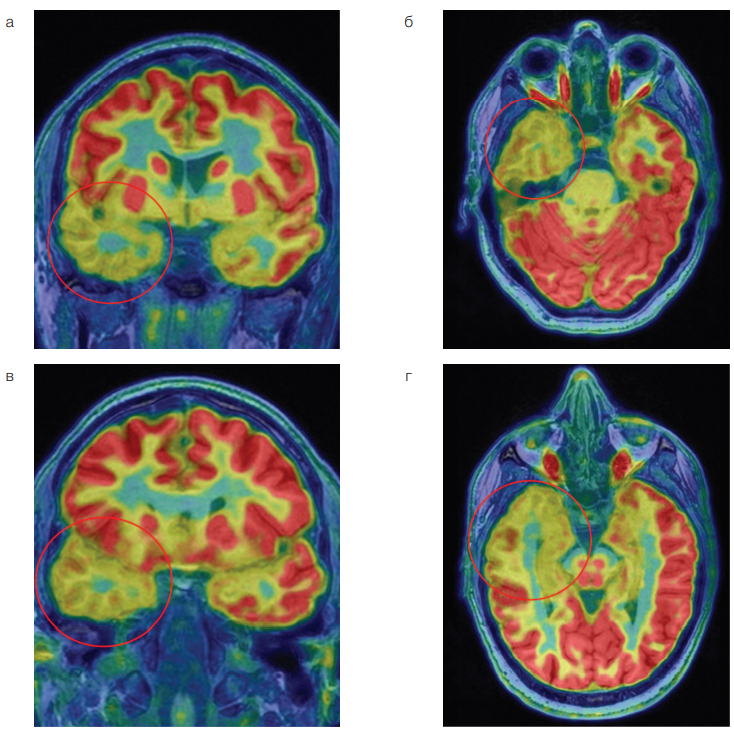
Figure prepared by the authors based on their own data
Fig. 2. PET/MRI of the brain in a patient with bilateral mesial temporal sclerosis
Note: a — combined PET and T1-weighted images in the coronal plane; b — combined PET and T1-weighted images in the axial plane; c — combined PET and T1-weighted images in the oblique coronal plane; d — combined PET and T1 weighted images in the oblique axial plane. The mark indicates the area of hypometabolism 18F-FDG in the right temporal lobe.
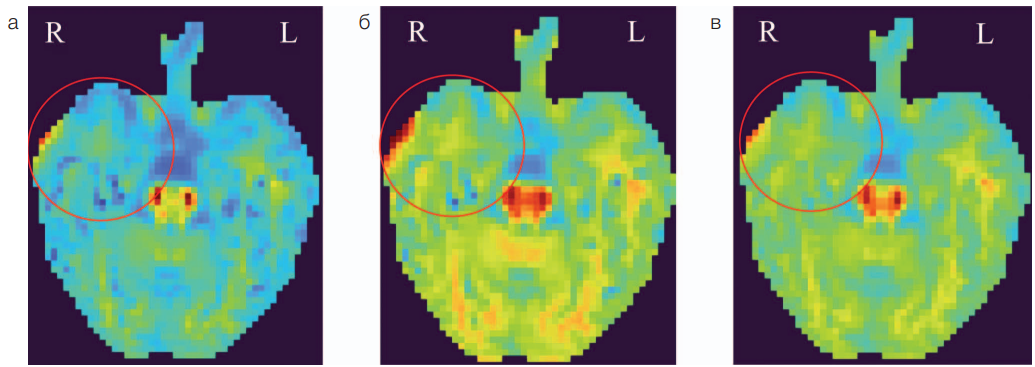
Figure prepared by the authors based on their own data
Fig. 3. Parametric DKI maps in a patient with focal epilepsy and bilateral mesial temporal sclerosis
Note: a — parametric map of radial kurtosis; b — parametric map of average kurtosis; c– parametric map of axonal water fraction. The mark indicates the zone of decrease in DKI parameters in the right temporal lobe; R — right hemisphere; L — left hemisphere.
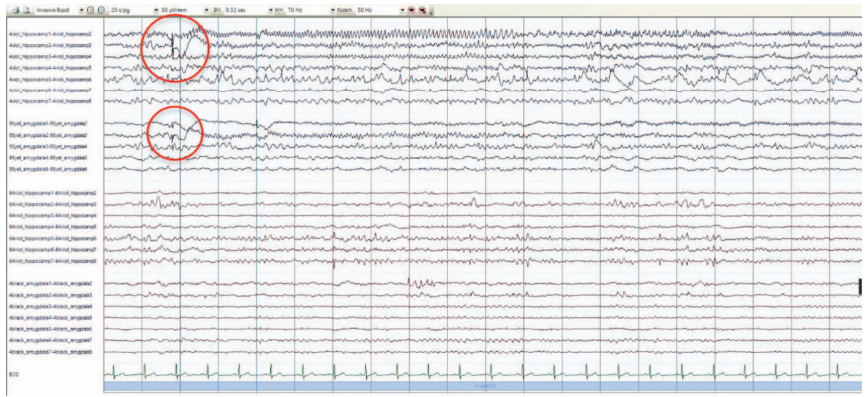
Figure prepared by the authors based on their own data
Fig. 4. Results of invasive EEG monitoring
Note: the markers show the zones of the onset of an electrographic attack.
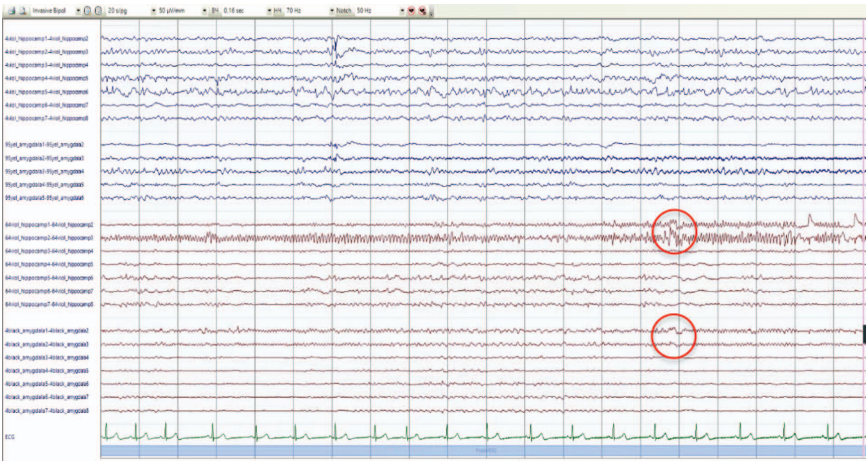
Figure prepared by the authors based on their own data
Fig. 5. Results of invasive EEG monitoring
Note: the markers show the zones of the onset of an electrographic attack.
CLINICAL CASE DISCUSSION
Localization of an EF is a challenging task in 30% of patients with pharmacoresistant focal epilepsy [9]. This is related to the capacity of brain MRI based on the epileptic protocol to detect macrostructural, rather than functional, changes. Nevertheless, MRI analysis may reveal structural changes in multiple locations, including in different hemispheres [10]. Such structural changes may manifest secondary epileptogenesis, which is frequently observed in the contralateral hemisphere [11]. Secondary EFs also have epileptogenic activity; therefore, resection surgery in some of such patients seems ineffective and impractical [12]. However, the presented clinical case shows that the latest non-invasive imaging techniques in the form of PET/MRI and DKI can provide additional information about the nature of changes in potential EFs, as well as their contribution to the course of epilepsy in a particular patient. This combined approach may be effective in patients with discordant results of continued EEG monitoring and conventional brain MRI according to the epileptic protocol, which was noted in the described patient. The DKI parameters make it possible to assess microstructural changes in the brain matter that cannot be reflected on conventional MRI sequences [13][14]. In turn, functional and metabolic changes caused by microstructural damage can be assessed by PET/MRI [15]. According to literature sources, the PET/MRI method demonstrates a higher sensitivity in the diagnosis of structural focal epilepsy compared to PET, PET/CT, and MRI scans [18].
In the presented case, the revealed changes indicate a more pronounced microstructural damage to the substance of the right temporal lobe, which can be interpreted as potential markers of more active EF or primary, longer-existing EF. The patient was discharged with recommendations for continued therapy and observation at his place of residence. In the future, hospitalization at the Federal Center of Brain Research and Neurotechnologies is planned for subsequent monitoring and determination of treatment tactics.
CONCLUSION
The combined use of PET/MRI and DKI extends the possibilities of pre-surgical diagnostics of patients with focal epilepsy, particularly in cases with potential multiple epileptogenic foci. These techniques provide a multimodal assessment of pathological changes, combining data on metabolic disorders (PET) and microstructural features of tissue (DKI), thus allowing identification of even subclinical or morphologically undetectable epileptogenic zones. The integration of the as-obtained results contributes to differentiation of primary and secondary foci, determination of their functional activity, and optimization of treatment tactics. This is especially important when planning a surgical intervention. In addition, identification of the most active epileptogenic sites can also influence the management of such patients. Thus, PET/MRI and DKI in combination with conventional methods increase the accuracy of topical diagnostics and contribute to personalized treatment of pharmacoresistant epilepsy. Another promising research direction is the development of algorithms for automated data analysis to standardize the diagnostic process.
References
1. Rugg-Gunn F, Miserocchi A, McEvoy A. Epilepsy surgery. Practical Neurology. 2020;20:4–14. https://doi.org/10.1136/practneurol-2019-002192
2. Mula M, Cock HR. More than seizures: improving the lives of people with refractory epilepsy. Eur J Neurol. 2015;22(1):24–30 https://doi.org/10.1111/ene.12603
3. Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. https://doi.org/10.1016/s0140-6736(18)32596-0
4. de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–95 https://doi.org/10.1016/s0140-6736(11)60890-8
5. Mathon B, Bielle F, Samson S, Plaisant O, Dupont S, Bertrand A, Miles R, Nguyen-Michel VH, Lambrecq V, Calderon-Garcidueñas AL, Duyckaerts C, Carpentier A, Baulac M, Cornu P, Adam C, Clemenceau S, Navarro V. Predictive factors of long-term outcomes of surgery for mesial temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsia. 2017;58(8):1473–85. https://doi.org/10.1111/epi.13831
6. Felix Rosenow, Hans Lüders. Presurgical evaluation of epilepsy. Brain. 2001;124(9):1683–700. https://doi.org/10.1093/brain/124.9.1683
7. Männlin J, San Antonio-Arce V, Reinacher PC, Scheiwe C, Shah MJ, Urbach H, Schulze-Bonhage A. Safety profile of subdural and depth electrode implantations in invasive EEG exploration of drug-resistant focal epilepsy. Seizure. 2023;110:21–7. https://doi.org/10.1016/j.seizure.2023.05.022
8. Dolgushin MB, Nadelyaev RV, Martynov MYu, Rubleva YuV, Dvoryanchikov AV, Burd SG, et al. Brain microstructural abnormalities assessed by diffusion kurtosis MRI in patients with focal temporal lobe epilepsy. S.S. Korsakov Journal of Neurology and Psychiatry. 2024;124(11):171–7 (In Russ.). https://doi.org/10.17116/jnevro2024124111171
9. Kanber B, Vos SB, de Tisi J, et al. Detection of covert lesions in focal epilepsy using computational analysis of multimodal magnetic resonance imaging data. Epilepsia. 2021;62(3):807–16 https://doi.org/10.1111/epi.16836
10. Iwasaki M, Jin K, Nakasato N, Tominaga T. Non-invasive Evaluation for Epilepsy Surgery. Neurol Med Chir. 2016;56(10):632–40. https://doi.org/10.2176/nmc.ra.2016-0186
11. Shen Y, Gong Y, Ruan Y, Chen Z, Xu C. Secondary Epileptogenesis: Common to See, but Possible to Treat? Front Neurol. 2021;12:747372. https://doi.org/10.3389/fneur.2021.747372
12. Dalkilic EB. Neurostimulation Devices Used in Treatment of Epilepsy. Curr Treat Options Neurol. 2017;19(2):7 https://doi.org/10.1007/s11940-017-0442-9
13. Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol. 2014;202(1):W26–33. https://doi.org/10.2214/ajr.13.11365
14. Bartoňová M, Bartoň M, Říha P, Vojtíšek L, Brázdil M, Rektor I. The benefit of the diffusion kurtosis imaging in presurgical evaluation in patients with focal MR-negative epilepsy. Sci Rep. 2021;11(1):14208. https://doi.org/10.1038/s41598-021-92804-w
15. Miller-Thomas MM, Benzinger TL. Neurologic Applications of PET/MR Imaging. Magn Reson Imaging Clin N Am. 2017;25(2):297–313. https://doi.org/10.1016/j.mric.2016.12.003
16. Jehi L. The Epileptogenic Zone: Concept and Definition. Epilepsy Curr. 2018;18(1):12–6. https://doi.org/10.5698/1535-7597.18.1.12
17. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43(3):219–27. https://doi.org/10.1046/j.1528-1157.2002.26901.x
18. Tóth M, Barsi P, Tóth Z. The role of hybrid FDG-PET/MRI on decision-making in presurgical evaluation of drug-resistant epilepsy. BMC Neurol. 2021;21(1):363. https://doi.org/10.1186/s12883-021-02352-z
19. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021
About the Authors
R. V. NadelyaevRussian Federation
Rostislav V. Nadelyaev
Moscow
M. B. Dolgushin
Russian Federation
Mikhail B. Dolgushin, Dr. Sci. (Med.)
Moscow
T. M. Rostovtseva
Russian Federation
Tatiana M. Rostovtseva
Moscow
E. A. Baranova
Russian Federation
Elena A. Baranova, Cand. Sci. (Med.)
Moscow
Yu. A. Voronkova
Russian Federation
Yulia A. Voronkova
Moscow
E. Yu. Gavrilova
Russian Federation
Elmira Yu. Gavrilova
Moscow
Yu. V. Rubleva
Russian Federation
Yulia V. Rubleva
Moscow
Supplementary files
Review
For citations:
Nadelyaev R.V., Dolgushin M.B., Rostovtseva T.M., Baranova E.A., Voronkova Yu.A., Gavrilova E.Yu., Rubleva Yu.V. Hybrid imaging techniques in the assessment of epileptic foci: A clinical case. Extreme Medicine. 2025;27(2):176-182. https://doi.org/10.47183/mes.2025-240
















