Scroll to:
Bone metabolism markers in young high-performance athletes
https://doi.org/10.47183/mes.2025-256
Abstract
Introduction. When assessing bone metabolism markers in athletes under the age of 18, it should be borne in mind that, in comparison with adults, the pediatric population is characterized by higher values of these markers. Their maximum increase during puberty coincides with peak bone mass gain.
Objective. To evaluate bone metabolism status in healthy high-performance athletes under the age of 18 based on the levels of C-terminal telopeptide (β-CrossLaps), osteocalcin, and N-terminal propeptide human procollagen type 1 (P1NP) in the blood serum.
Materials and methods. A single-center, сross-sectional study involved 383 juvenile athletes aged 13–18 years (248 girls and 135 boys; average age 15.2 [14.0; 16.1] years) from Russian national sports teams. The study was conducted in the period from March 2021 to July 2023. All athletes were divided into groups according to age and gender. The male groups were as follows: 13.1–14.0 years old (n = 3); 14.1–15.0 years old (n = 11); 15.1–16.0 years old (n = 43); 16.1–17.0 years old (n = 42); and 17.1–18.0 years old (n = 36). The female groups were as follows: 13.1–14.0 years old (n = 17); 14.1–15.0 years old (n = 51); 15.1–16.0 years old (n = 65); 16.1–17.0 years old (n = 59); and 17.1–18.0 years old (n = 56). The serum levels of osteocalcin, C-terminal telopeptide, and procollagen type 1 were evaluated in all athletes. The sexual maturity rating (SMR) was assessed according to the Tanner Scale. Statistical data processing was performed using the Statistica 10.0 software package (StatSoft Inc.; USA).
Results. The maximum values of β-CrossLaps in boys (2.27 [1.14; 3.45] ng/mL) and girls (1.55 [1.10; 2.02] ng/mL) were observed at the age of 13–14 years. The levels of osteocalcin and P1NP in young high-performance athletes corresponded to the standards for children with a normal level of physical activity. The maximum values of P1NP were revealed at the age of 13–14 years in both male (767.8 [148.1; 1142.4] ng/ mL) and female (450.5 [268.6; 569.3] ng/mL) groups. In boys, the maximum values of osteocalcin (125 [89; 144] ng/mL) were detected at the age of 14–15 years; in girls (86 [62; 131] ng/mL) — at the age of 13–14 years.
Conclusions. In young high-performance athletes, the β-CrossLaps level as the main marker of bone resorption significantly exceeds the population norms for children and adolescents with a normal level of physical activity. When assessing the level of β-CrossLaps, osteocalcin, and P1NP, reference values should be adjusted to account for the gender and sexual maturity stage of athletes. The data obtained can be used when interpreting the results of an in-depth medical examination of athletes from Russian national sports teams to identify bone remodeling disorders.
For citations:
Isaeva E.P., Okorokov P.L., Stolyarova S.A., Kluchnikov S.O., Zyabkin I.V., Isaev M.R., Fechenko V.S. Bone metabolism markers in young high-performance athletes. Extreme Medicine. 2025;27(3):384-391. https://doi.org/10.47183/mes.2025-256
INTRODUCTION
Assay of bone metabolism markers is an effective diagnostic tool for assessing the functional status of the skeletal system in clinical practice [1–4]. However, high growth rates in children (especially in adolescents) intensify the processes of bone metabolism and, compared to adults, are associated with higher values of bone metabolism markers. Intense and prolonged physical activity performed by high-performance athletes can also affect the level of these markers [5][6]. The syndrome of relative energy deficiency in sport (RED-S) is associated with a decreased acquisition of bone mass and bone microarchitectonics disorders in adolescence [2–4]. The hypothalamic amenorrhea (for girls) and functional hypogonadotropic hypogonadism (for boys), as part of RED-S, combined with vitamin D deficiency, is an additional risk factor for fractures in professional athletes, especially under the age of 18 [2][5–8].
In the Russian Federation, research is currently underway to determine regulatory values for a number of biochemical laboratory parameters in juvenile high-performance athletes [9][10]. In this study, we aim to evaluate the bone metabolism status of healthy high-performance athletes under the age of 18 based on the levels of β-CrossLaps, osteocalcin, and P1NP in the blood serum.
MATERIALS AND METHODS
A single-center, cross-sectional study involved young athletes from the national teams of the Russian Federation who had underwent an in-depth medical examination at the Federal Scientific and Clinical Center for Children and Adolescents in the period from March 2021 to July 2023. A total of 383 young athletes aged 13–18 years participated in the study, including 248 girls and 135 boys; the average age was 15.2 [ 14.0; 16.1] years. All athletes were divided into groups according to gender and age. The male groups were as follows: 13.1–14.0 years old (n = 3), 14.1–15.0 years old (n = 11), 15.1–16.0 years old (n = 43), 16.1–17.0 years old (n = 42), and 17.1–18.0 years old (n = 36). The female groups were as follows: 13.1–14.0 years old (n = 17), 14.1–15.0 years old (n = 51), 15.1–16.0 years old (n = 65), 16.1–17.0 years old (n = 59), and 17.1–18.0 years old (n = 56).
According to sexual maturity, athletes were distributed as follows: 5 (1.3%) athletes did not enter puberty, 17 (4.4%) — stage 2 sexual maturity, 57 (14.8%) athletes — stage 3, 174 (45.4%) athletes — stage 4 sexual maturity, the remaining 130 athletes had reached sexual maturity. The sexual maturity assessment was carried out according to the Tanner Scale (Sexual Maturity Rating, SMR) [11]. The key inclusion criteria were athletes from Russian national teams aged 13–18 years. The exclusion criteria were the presence of fractures during one year prior to inclusion in the study.
For clinical and laboratory tests, peripheral vein blood was collected in the morning after fasting. In all young athletes, the levels of osteocalcin (Roche, Switzerland), N-terminal propeptide human procollagen type 1/P1NP (Roche, Switzerland), and C-terminal telopeptide/β-CrossLaps (Roche, Switzerland) in blood serum (ng/mL) were evaluated. The β-CrossLaps assay was performed by electrochemiluminescence using a Cobase411 analyzer (Roche Diagnostics, Germany). The level of N-terminal propeptide human procollagen type 1 (P1NP) and osteocalcin was detected by enzyme-linked immunosorbent assay (ELISA). The P1NP level was assessed by reference intervals proposed by Chubb et al. [12]. The osteocalcin level was evaluated by reference intervals proposed by Bayer et al. [13]. The level of β-CrossLaps was calculated according to the reference intervals proposed by Crofton et al. [14].
Statistical data processing was performed using the Statistica 10.0 software package (StatSoft Inc.; USA). Since the parameters under study showed a non-normal distribution (according to the Kolmogorov–Smirnov test), all the data are presented as the median (Me) of both the first and third quartiles [Q1; Q3]. To assess the statistical significance of the differences in quantification, the Mann–Whitney and Kruskall–Wallis tests were used, including with the Bonferroni adjustment. Qualitative characters are presented in proportions (%) with absolute values. To assess the differences between qualitative characters, contingency tables are charted with subsequent evaluation according to the Pearson’s chi-squared test. The statistical significance of the differences was p < 0.05.
RESULTS
The levels of β-CrossLaps in athletes under the age of 18, depending on age and gender, were found to be statistically significantly higher in boys (p < 0.01) (Table 1). The established gender differences in the β-CrossLaps level are most likely to be associated with the larger bone mass accrual in boys compared to girls. The maximum values of β-CrossLaps in boys (2.27 [ 1.14; 3.45] ng/mL) and girls (1.55 [ 1.10; 2.02] ng/mL) are reached at the age of 13–14 years.
When assessing the β-CrossLaps levels in athletes under the age of 18, compared with the reference intervals proposed for children by Crofton et al. [14], significant differences were found, manifested in an increase in this parameter in athletes compared with its values in children in the general pediatric population, regardless of gender and age (Fig. 1).
Figure 1 shows that most of the individual β-CrossLaps values in both boys and girls exceeded the upper limit of the reference interval.
The maximum values of β-CrossLaps were determined in young athletes with stage 3 sexual maturity at the level of 2.31 [1.91; 3.4] ng/mL in boys and 1.98 [ 1.51; 2.31] ng/mL in girls (Table 2). Such changes are associated with active bone metabolism during peak growth and accrual of muscle tissue in adolescents.
When assessing osteocalcin levels in athletes under the age of 18, statistically significantly higher osteocalcin levels were found in boys, depending on gender, compared to girls in all age groups (Table 3). The maximum values of osteocalcin in boys (125 [ 89; 144] ng/mL) are revealed at the age of 14–15 years old; in girls (86 [ 62; 131] ng/mL) — at the age of 13–14 years, which is also due to the earlier onset of puberty in girls.
Osteocalcin levels in athletes under the age of 18 did not exceed the limits of the reference interval [13] (Fig. 2). As shown in Figure 2, most of the individual values of osteocalcin in both boys and girls remained within the reference interval.
When assessing the levels of P1NP in athletes under the age of 18, statistically significantly higher levels of P1NP were found in boys, depending on gender, compared to girls in all age groups (Table 4). The maximum values of P1NP were determined at the age of 13–14 years, in both boys (767.8 [ 148.1; 1142.4] ng/mL) and girls (450.5 [ 268.6; 569.3] ng/mL).
When assessing the levels of P1NP in athletes under the age of 18, compared to the reference intervals proposed by Chubb et al. for children [12], no gender differences were found (Fig. 3). As shown in Figure 3, most of the individual P1NP values in both boys and girls did not exceed the reference values.
When assessing the levels of osteocalcin and P1NP depending on the stage of sexual maturity according to the Tanner Scale, the maximum values of osteocalcin (102 [ 77; 131] ng/mL) were revealed in athletes with stage 3 sexual maturity, and the maximum level of P1NP (642.3 [ 537.9; 789.3] ng/mL) — in athletes with stage 2 sexual maturity (Table. 5). These data correlate with the growth rate peak value in adolescents.
Table 1. β-CrossLaps levels in athletes under the age of 18, depending on gender and age
|
Age, years |
Boys |
Girls |
Statistical significance level, p |
||||
|
Median [Q1; Q3] |
Min |
Max |
Median [Q1; Q3] |
Min |
Max |
||
|
13.1–14.0 |
2.27 [ 1.14; 3.45] n = 3 |
0.330 |
5.300 |
1.55 [ 1.10; 2.02] n = 17 |
0.480 |
2.52 |
< 0.01 |
|
14.1–15.0 |
2.21 [ 1.64; 2.47] n = 11 |
0.510 |
4.450 |
1.420 [ 1.19; 1.68] n = 51 |
0.690 |
2.77 |
< 0.01 |
|
15.1–16.0 |
1.46 [ 1.11; 2.00] n = 43 |
0.520 |
3.080 |
1.10 [ 0.88; 1.40] n = 65 |
0.480 |
4.03 |
< 0.01 |
|
16.1–17.0 |
1.20 [ 0.68; 1.72] n = 42 |
0.320 |
4.140 |
0.92 [ 0.65; 1.09] n = 59 |
0.310 |
2.46 |
< 0.01 |
|
17.1–18.0 |
1.25 [ 0.87; 1.75] n = 36 |
0.320 |
2.660 |
0.86 [ 0.53; 1.09] n = 56 |
0.250 |
5.90 |
< 0.01 |
Table compiled by the authors based on their own data
Note: n — number of athletes.
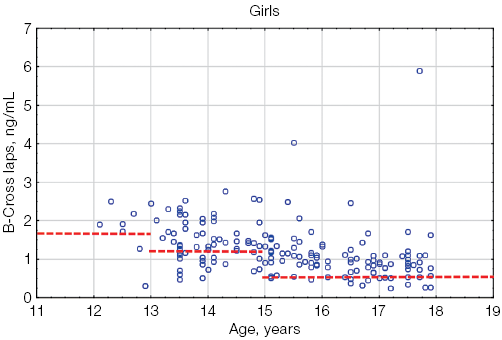
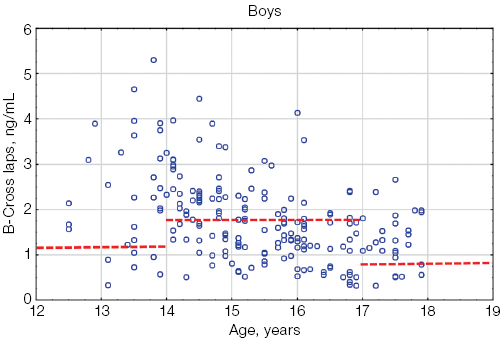
Figure prepared by the authors based on their own data
Fig. 1. β-CrossLaps values in athletes under the age of 18 in comparison with the general pediatric reference intervals depending on age: the red dotted lines indicate the upper limit of the general pediatric reference intervals of β-CrossLaps according to [14] for boys and girls, taking into account age; the blue circles represent the individual values of β-CrossLaps for each athlete; n — number of athletes
Table 2. β-CrossLaps levels in athletes under the age of 18, depending on gender and sexual maturity stage
|
Sexual maturity rating (Tanner stages) |
1 |
2 |
3 |
4 |
5 |
|
Boys |
1.57 [ 1.34; 1.74] n = 1 |
2.14 [ 1.97; 2.51] n = 11 |
2.31 [ 1.91; 3.4] n = 17 |
2.14 [ 1.64; 2.65] n = 56 |
1.45 [ 1.23; 1.88] n = 50 |
|
Girls |
1.29 n = 4 |
1.87 [ 1.78; 2.30] n = 6 |
1.98 [ 1.51; 2.31] n = 40 |
1.33 [ 0.96; 1.56] n = 118 |
1.22 [ 0.98; 1.29] n = 80 |
Table compiled by the authors based on their own data
Note: n — number of athletes.
Table 3. Osteocalcin levels in athletes under the age of 18, depending on gender and age
|
Age, years |
Boys |
Girls |
Statistical significance level, p |
||||
|
Median [Q1; Q3] |
Min |
Max |
Median [Q1; Q3] |
Min |
Max |
||
|
13.1–14.0 |
113 [ 88; 155] n = 3 |
88.0 |
155.0 |
86 [ 62; 131] n = 17 |
36.0 |
229.0 |
– |
|
14.1–15.0 |
125 [ 89; 144] n = 11 |
77.0 |
201.0 |
68 [ 49; 98] n = 51 |
17.0 |
145.0 |
0.013 |
|
15.1–16.0 |
78 [ 63; 106] n = 43 |
13.0 |
190.0 |
58 [ 43; 71] n = 65 |
28.0 |
137.0 |
0.034 |
|
16.1–17.0 |
68 [ 53; 90] n = 42 |
28.0 |
174.0 |
44 [ 35; 61] n = 59 |
24.0 |
110.0 |
0.027 |
|
17.1–18.0 |
51 [ 42; 63] n = 36 |
10.0 |
110.0 |
36 [ 31; 49] n = 56 |
23.0 |
146.9 |
0.021 |
Table compiled by the authors based on their own data
Note: n — number of athletes; “–” — non-significant statistical results.
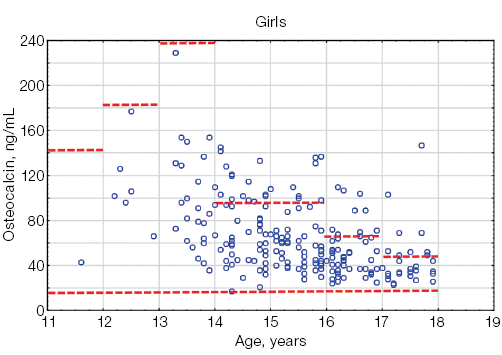
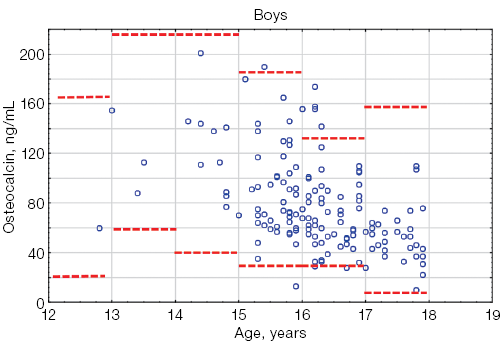
Figure prepared by the authors based on their own data
Fig. 2. Osteocalcin levels in athletes under the age of 18 compared to the general pediatric reference intervals depending on gender: the red dotted lines indicate the upper and lower boundaries of the general pediatric reference intervals [13] for boys and girls, taking into account age; the blue circles represent individual osteocalcin values for each athlete; n — number of athletes; “–” — non-significant statistical results
Table 4. P1NP levels in athletes under the age of 18, depending on gender and age
|
Age, years |
Boys |
Girls |
Statistical significance level, p |
||||
|
Median [Q1; Q3] |
Min |
Max |
Median [Q1; Q3] |
Min |
Max |
||
|
13.1–14.0 |
767.8 [ 148.1;1142.4] n = 3 |
148.1 |
1142.4 |
450.5 [ 268.6; 569.3] n = 17 |
128.2 |
1324.0 |
– |
|
14.1–15.0 |
689.9 [ 548.4;727.7] n = 11 |
446.0 |
1398.0 |
250.2 [ 209.0;599.6] n = 51 |
75.3 |
1053.0 |
0.023 |
|
15.1–16.0 |
425.1 [ 300.7;662.2] n = 43 |
127.1 |
1298.2 |
239.0 [ 167.1;369.0] n = 65 |
85.8 |
838.1 |
0.034 |
|
16.1–17.0 |
288.8 [ 219.4;473.9] n = 42 |
115.2 |
1155.0 |
164.5 [ 119.6;228.7] n = 59 |
78.9 |
611.1 |
0.017 |
|
17.1–18.0 |
227.0 [ 182.4;278.3] n = 36 |
95.1 |
680.2 |
138.0 [ 106.3;188.8] n = 56 |
39.0 |
335.1 |
0.022 |
Table compiled by the authors based on their own data
Note: n — number of athletes.
Table 5. Osteocalcin and P1NP levels in athletes under the age of 18, depending on sexual maturity rating
|
Sexual maturity rating (Tanner stages) |
1 |
2 |
3 |
4 |
5 |
|
Number of athletes, n |
5 |
17 |
57 |
174 |
130 |
|
Osteocalcin |
60 [ 46; 88] |
97 [ 89; 121] |
102 [ 77; 131] |
62 [ 44; 81] |
49 [ 37; 65] |
|
P1NP |
296.1 [ 153.1;459.7] |
642.3 [ 537.9;789.3] |
605.3 [ 432.1;769.7] |
243.6 [ 176.2;408.1] |
200.9 [ 149.4;270.0] |
Table compiled by the authors based on their own data
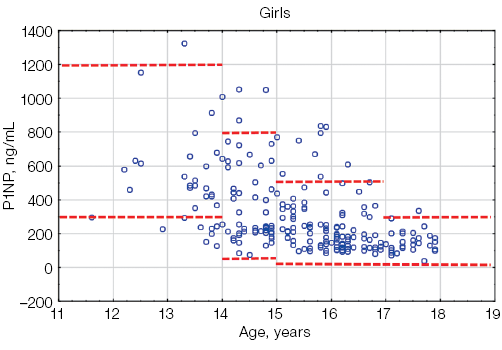
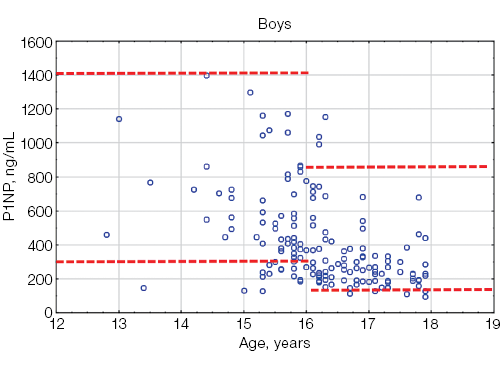
Figure prepared by the authors based on their own data
Fig. 3. P1NP values in athletes under the age of 18 compared with the general pediatric reference intervals depending on gender: the red dotted lines indicate the upper and lower limits of the general pediatric reference intervals [12] for boys and girls, taking into account age; the blue circles represent individual P1NP values for each athlete; n — number of athletes
DISCUSSION
β-CrossLaps is the most informative marker of bone resorption that indicates the activity of osteoclasts. This marker is widely used in clinical pediatric practice and is included in medical examination programs of young athletes. Permanently elevated β-CrossLaps levels in adult athletes indicate their prolonged exposure to high-intensity loads and inconsistency with their overall physical fitness level, which can lead to chronic overexertion or microtrauma, disrupting the structure and function of bone tissue [15]. Assessment of β-CrossLaps levels in young high-performance athletes revealed an increase in this parameter in all age groups, particularly in 14–15-year-old athletes. The average values of β-CrossLaps in athletes are two- or threefold higher than the standard values for people with a daily level of physical activity [15]. The authors explain the increased level of this marker by the specific nature of anabolic processes in juvenile athletes. Establishing a correlation with the type of athletic discipline, Klyuchnikov et al. found the maximum values of β-CrossLaps in athletes engaged in game sports, with the average values of β-CrossLaps in boys being higher than those in girls [15].
The results of our work also demonstrate the previously described gender differences and the fact that the β-CrossLaps level in young athletes is higher than that in the general pediatric population.The maximum values of β-CrossLaps in young athletes are recorded at the 2nd–3rd stage of puberty, which, according to the literature [14], coincides with the peak height velocity and is associated with the peak bone mass gain. Thus, the increased activity of bone resorption detected in young athletes compared with their peers with normal physical activity may be due to the intensity and nature of physical exertion.
Osteocalcin is a non-collagen protein of the bone matrix, synthesized by osteoblasts. This parameter reflects the osteosynthesis activity. Osteocalcin levels gradually increase in childhood, reaching their maximum values in puberty. The osteocalcin concentration in children correlates with the height velocity and increases progressively during puberty, regardless of gender. The maximum levels of this biomarker are recorded at the age of 13–14 years, ranging 25–241 ng/mL [13].
Our study has demonstrated that the maximum level of osteocalcin is recorded in girls in the age group of 13–14 years, in boys — in the age group of 14–15 years. Osteocalcin values increase with the progression of sexual development from stage 1 to stage 2, followed by a decrease by the end of puberty. In children and adolescents, more than 90% of the synthesized osteocalcin is incorporated into the bone matrix, with only its small part circulating in the circulatory system. In addition, the level of osteocalcin is subject to pronounced daily fluctuations; therefore, the study should be conducted in the morning. Osteocalcin levels in young athletes are higher than those in adults [5].
The N-terminal propeptide of human procollagen type 1 is a marker of bone matrix formation. The variability and higher levels of P1NP in childhood are due to the active processes of child growth and development. The acceleration of height velocity in early childhood and during sexual maturation is accompanied by a significant increase in the P1NP level. The maximum values of this biomarker in boys are recorded in the first year of life, reaching the values of 3000 ng/mL, with a gradual decrease to 950 ng/mL by the age of 11. In the 11–16-year group, a repeated increase in the P1NP level to 1400 ng/mL was observed. The lower limit of P1NP in this age group in boys is 300 ng/mL. In girls, the maximum values of P1NP are also recorded in the first year of life (600–3000 ng/mL) with a gradual decrease by the age of 9 years. From 9 to 14 years of age, serum P1NP levels continue to rise (the standard range is 300–1200 ng/mL) [12]. The described gender-specific features of P1NP secretion in childhood are due to the different timing of the onset of sexual maturation in boys and girls. Thus, the maximum values of P1NP in boys are recorded at stage 3 sexual maturity according to the Tanner scale; in girls — at stage 2 of sexual maturity according to the Tanner Scale [12].
Our study has also demonstrated that the maximum levels of P1NP in young athletes are observed at the age of 13–15 years, with a further gradual decrease towards the end of sexual maturation.
Thus, the levels of bone synthesis markers in young athletes correlate with the reference intervals for the general pediatric population, with their variability in childhood being due to increased metabolism in bone tissue during the period of active growth.
An important limitation of this study is the lack of information at the time of blood sampling about the intake of colecalciferol and other biologically active additives that affect bone metabolism. In addition, the study did not evaluate the discipline-related effect on the studied bone metabolism markers due to the limited sample size of athletes. Nevertheless, the obtained clinical results demonstrate a tendency to higher values of osteocalcin and P1NP in athletes engaged in complex coordination sports. This aspect requires additional elucidation, which can be of practical importance for developing an individualized approach to interpretation of laboratory parameters.
CONCLUSION
The level of β-CrossLaps, the main marker of bone resorption, in young high-performance athletes was found to be significantly higher than the population norms for children and adolescents with a normal level of physical activity. When assessing the β-CrossLaps level in athletes under the age of 18, reference values based on gender and sexual maturity stage should be used. The β-CrossLaps levels in girls are statistically significantly lower than those in boys, which may be due to larger bone and muscle mass in males.
The reference values of osteocalcin and P1NP in young athletes correlate with the values recorded in the general pediatric population. However, when assessing osteocalcin and P1NP levels in athletes under the age of 18, reference values should be adjusted for the stage of sexual maturity, since these bone remodeling markers are characterized by a physiological increase against the background of accelerated height velocity during sexual maturation.
The data obtained can be used when interpreting the results of an in-depth medical examination of athletes from Russian national sports teams to identify bone remodeling disorders and form individual prevention and correction programs within the framework of medical and biological support for high-performance sports.
References
1. Kiseleva NG, Taranushenko TE, Golubenko NK. Diagnosis of osteoporosis at an early age. Medical Council. 2020;(1):179–86 (In Russ.). https://doi.org/10.21518/2079-701X-2020-1-186-193
2. Mountjoy M, Ackerman KE, Bailey DM, Burke LM, Constantini N, Hackney AC, et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). British Journal of Sports Medicine. 2023;57(17):1073–97. https://doi.org/10.1136/bjsports-2023-106994
3. Mountjoy M, Sundgot-Borgen J, Burke L, Ackerman KE, Blauwet C, Constantini N, et al. International Olympic Committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. International journal of sport nutrition and exercise metabolism. 2018;28(4):316–31. https://doi.org/10.1123/ijsnem.2018-0136
4. Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121(6):1127–36. https://doi.org/10.1542/peds.2007-2392
5. Kaga M, Takahashi K, Ishihara T, Suzuki H, Tanaka H, Seino Y, et al. Bone assessment of female long-distance runners. Journal of Bone and Mineral Metabolism. 2004;22(5):509–13. https://doi.org/10.1007/s00774-004-0515-1
6. Hackney AC, Constantini NW. Endocrinology of Physical Activity and Sport. 3 rd ed. Cham: Humana press;2020. https://doi.org/10.1007/978-3-030-33376-8
7. Yang G, Lee WYW, Hung ALH, Tang MF, Li X, Kong APS, et al. Association of serum 25(OH)Vit-D levels with risk of pediatric fractures: a systematic review and meta-analysis. Osteoporosis International. 2021;32(7):1287–1300. https://doi.org/10.1007/s00198-020-05814-1
8. Isaeva EP, Okorokov PL, Zyabkin IV. Secondary hyperparathyroidism associated with vitamin D deficiency in young highly trained athletes. Extreme Medicine. 2024; 26(2):76–82 (In Russ.). https://doi.org/10.47183/mes.2024.033
9. Grishina ZhV, Klyuchnikov SO, Feshchenko VS, Zholinskiy AV. Calculation of reference intervals of blood parameters in children and adolescents: projects review. Extreme Medicine. 2023;25(1):4–11 (In Russ.). https://doi.org/10.47183/mes.2023.008
10. Grishina ZhV, Klyuchnikov SO, Feshchenko VS, Zholinsky AV, Okorokov PL. Reference ranges of biochemical blood parameters in juvenile athletes. Rossiyskiy Vestnik Perinatologii i Pediatrii. 2022;67(4):60–68 (In Russ.). https://doi.org/10.21508/1027-4065-2022-67-4-60-68
11. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of disease in childhood. 1976;51(3):170. https://doi.org/10.1136/adc.51.3.170
12. Chubb SAP, Vasikaran SD, Gillett MJ. Reference intervals for plasma β-CTX and P1NP in children: A systematic review and pooled estimates. Clinical Biochemistry. 2023;(118):110582. https://doi.org/10.1016/j.clinbiochem.2023.05.001
13. Bayer M. Reference values of osteocalcin and procollagen type I N-propeptide plasma levels in a healthy Central European population aged 0–18 years. Osteoporosis International. 2014;25(2):729–36. https://doi.org/10.1007/s00198-013-2485-4
14. Crofton PM, Evans N, Taylor MR, Holland CV. Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clinical Chemistry. 2002;48(4):671–3. http://dx.doi.org/10.1093/clinchem/48.4.671
15. Kljuchnikov SO, Kravchuk DA, Ogannisyan MG. Osteoporosis and its actuality for child sports medicine. Russian Bulletin of Perinatology and Pediatrics. 2017; 62:(3):112–20 (in Russ.). https://doi.org/10.21508/1027-4065-2016-61-3-112-120
16.
About the Authors
E. P. IsaevaRussian Federation
Elena P. Isaeva, Cand. Sci. (Med.)
Moscow
P. L. Okorokov
Russian Federation
Pavel L. Okorokov, Cand. Sci. (Med.)
Moscow
S. A. Stolyarova
Russian Federation
Svetlana A. Stolyarova, Cand. Sci. (Med.)
Moscow
S. O. Kluchnikov
Russian Federation
Sergey O. Klyuchnikov, Dr. Sci. (Med.)
Moscow
I. V. Zyabkin
Russian Federation
Ilya V. Zyabkin, Dr. Sci. (Med.)
Moscow
M. R. Isaev
Russian Federation
Maxim R. Isaev
Moscow
V. S. Fechenko
Russian Federation
Vladimir S. Feshchenko, Cand. Sci. (Med.)
Moscow
Supplementary files
Review
For citations:
Isaeva E.P., Okorokov P.L., Stolyarova S.A., Kluchnikov S.O., Zyabkin I.V., Isaev M.R., Fechenko V.S. Bone metabolism markers in young high-performance athletes. Extreme Medicine. 2025;27(3):384-391. https://doi.org/10.47183/mes.2025-256
















