Scroll to:
Integration prospects for the multiplex phosphorescence immunoassay of pooled dry urine samples into screening examinations in dispensary drug control
https://doi.org/10.47183/mes.2025-261
Abstract
Introduction. The worsening problem of drug abuse in Russia and the growing number of hidden users of narcotic drugs (ND) require the list of screening examinations for ND identification to be extended by including more economical approaches that reduce costs at the stages of collection, transportation, storage, and analytical examination of biological samples.
Objective. Development of a multiplex immunoassay method based on the PHOSPHAN technology for detecting the main groups of narcotic and psychotropic substances in pools of paper-dried urine samples, followed by an assessment of its potential for identifying drug addicts as part of an extended drug control program.
Materials and methods. Dry urine samples (n = 31) were prepared on paper test strips from liquid samples containing (n = 30) or non-containing (n = 1) cocaine, cannabinoids, amphetamines, opiates, benzodiazepines, barbiturates, methamphetamine, or methadone, according to toxicology screening (TS). The samples were studied as pools containing 1–40 fragments (0.45×0.45 cm) of test strips. The luminescent signal was recorded on a microplate immunochip using an IFI-05 photoluminescence pulsed indicator. The ND presence in the samples was assessed by the inhibition rate of antibody binding in the related microplate test zone (B/B0 ratio). Statistical processing of the results was carried out using the standard Microsoft Office package.
Results. The inclusion of dry urine samples in the pools (up to 10), where only one contained the target ND, had no significant effect on the capability of the method to detect NDs with sensitivity levels that meet the TS requirements. The following substances were detected: cocaine (2 samples), cannabinoids (11 samples), amphetamines (6 samples), opiates (9 samples), benzodiazepines (7 samples), barbiturates (10 samples), methamphetamine (7 samples), and methadone (6 samples), including samples with high concentrations of opiates and amphetamines.
Conclusions. A method of multiplex phosphorescence microplate immunoassay has been developed for the detection of eight main groups of NDs and psychotropic substances in pools of paper-dried urine samples (dried urine spot, DUS). The detection limits of the studied NDs in extracts from DUS test-strips were 2–8 ng/mL, which is significantly lower than the detection limits recommended for screening examination. The proposed approach can form the basis of a new screening methodology that includes collection of urine samples, their application onto filter paper test-strips, and transportation to a laboratory for the examination of individuals at industrial facilities of critical importance. The use of the developed multiplex phosphorescence immunoassay and pooled urine samples will significantly reduce the test cost (by more 10-fold) compared to conventional immunochromatographic assays.
Keywords
For citations:
Bekman N.I., Pomelova V.G., Osin N.S. Integration prospects for the multiplex phosphorescence immunoassay of pooled dry urine samples into screening examinations in dispensary drug control. Extreme Medicine. 2025;27(3):400-409. https://doi.org/10.47183/mes.2025-261
INTRODUCTION
Illicit use of narcotic drugs (ND) or their chemical analogues continues to be a global problem affecting approximately 292 million people (more than 5% of the world’s adult population)1. In Russia, approximately 2% of the population aged 15–64 use narcotic drugs2. This is due not only to the large-scale import of opium ND from abroad, but also to the appearance of new groups of narcotic and psychotropic substances (such as synthetic cannabinoids and spice drugs) and the continued involvement of young people in drug consumption [1]. The current drug situation in Russia is associated with an increasing number of hidden drug users. This group includes people aged 30–40 with a high level of education and social status, who intentionally consume “lighter” drugs. This contributes to the development of drug addiction with delayed acute periods, escaping the attention of law enforcement agencies or medical professionals [2].
Currently, medical examinations for potentially dangerous substances are conducted in cases specified by Russian legislation as part of a two-stage procedure for toxicology screening3. However, the worsening drug situation and the growing number of hidden drug users requires stricter screening examinations for drug detection through the organization of dispensary drug control. In addition, even a single drug use can affect the person’s ability to assess their surroundings and take appropriate actions in cases of emergency. Identification of drug addicts is particularly important considering the number of critically important and potentially dangerous infrastructure facilities in Russia, being directly related to ensuring the national security [3].
The organization of extended dispensary drug control requires significant financial expenditures. Thus, the cost of the most popular and affordable immunochromatographic test strips for detecting 6–8 main ND groups is at least 300 rubles, which practically excludes the possibility of introducing drug control at industrial enterprises through budget funding. The cost of testing one sample for 8–13 main ND groups should be significantly lower for screening purposes. New methodological approaches that have emerged in recent years, including the pooling of test samples, the use of clinical samples dried on special paper, the centralization of laboratory tests, and the introduction of multiplex assay technologies, could significantly (by more than 10–20 times) reduce the test cost by optimizing costs at the stages of collection, transportation, storage, and sample assay [4–10].
The pooling technique, i.e., the combination of samples from different people is a promising approach to dispensary drug control [4–10]. An obvious advantage of group testing consists in the reduction of test costs in proportion to the sample number in the pool, the total number of tests, and the minimization of dosing errors [7]. The effectiveness of this approach was demonstrated during the COVID-19 pandemic, when the number of required examinations increased progressively [8–10]. However, there are still doubts about the possibility of widespread use of group testing in laboratory practice due to the increased risk of missing infected patients and the possibility of sample confusion during additional manipulations associated with sample pooling procedures [7].
Taking the above into account, the following criteria [11] were proposed to assess the applicability of the pooling technique for detecting the analyte in question:
1) the analyte concentration in the test patients should be at least 10 times higher than in healthy individuals;
2) the sample dilution should not significantly reduce clinical sensitivity;
3) the prevalence of the test pathology should be low, within 1–10%;
4) the absence of requirements for test speed;
5) the need for resource rationing in order to achieve maximum efficiency in the measures taken based on the test results.
In our opinion, the methodology for detecting ND in pooled samples meets almost all of the above-mentioned criteria, which justifies its further development in combination with the use of highly sensitive multiplex tests.
Laboratory tests centralization also significantly reduces costs by collecting samples of biological material (urine, blood, and other body fluids) as a spot dried on filter paper, which are then transported to a central laboratory for assay [12, 13]. Postal delivery of test forms does not require a cold chain and can be used to accumulate samples in a single (or regional) diagnostic examination center, similarly to the procedures for screening hereditary metabolic diseases in newborns. The reliability of testing for biologically active compounds, including ND, in paper-dried samples has served as the basis for the introduction of this technology into the global system of the World Anti-Doping Agency (WADA)4 doping control of athletes.
Highly sensitive and cost-effective tools of detecting ND are required for the assay of pooled dry urine (or blood) samples. Conventional immunochromatographic assays are not suitable for this approach due to their low sensitivity and low accuracy. Gas chromatography–mass spectrometry shows higher sensitivity [14]; however, its potential for screening is limited by its high test cost and complexity.
A highly cost-effective solution is the development of a domestic technological platform for multiplex immunoassay based on microplate immunochips with time-resolved luminescent signal detection (PHOSPHAN™ technology) [15, 16]. Immunochips made using this technology are microarrays (microzones) at the bottom of 96-well microplates, each of which is designed to detect a specific type of neurotransmitter. The consumption of key reagents for creating such tests is reduced by many times compared to conventional immunochromatographic assays, while the microplate format of immunochips allows for parallel high-performance screening of multiple samples.
In this study, we aim to develop a multiplex immunoassay method based on the PHOSPHAN™ technology for detecting the main groups of narcotic and psychotropic substances in pools of paper-dried urine samples, followed by an assessment of its potential for identifying drug-addicted persons as part of extended drug control programs.
MATERIALS AND METHODS
The following immunobiologicals were used to create a test for detecting NDs: mouse monoclonal antibodies (MAb) to morphine (MOR), benzoylecgonine (BZE), amphetamine (AMP), methamphetamine (mAMP), methadone (MTD), benzodiazepine (BZD), barbiturates (BAR) and Δ9-tetra-hydrocannabinol (THC) (CALBIOREAGENTS Inc., USA), biotin-labeled according to the standard procedure (SIGMA, USA); conjugates of MOR, BZE, AMP, mAMP, MTD, BZD, BAR and THC with bovine serum albumin (CALBIOREAGENTS, USA).
A conjugate of streptavidin with Pt-coproporphyrin (Immunoscreen, Russia) was used as a detection reagent.
Dry samples for the study were prepared from 50 liquid human urine samples containing various narcotic and non-narcotic compounds in various combinations and concentrations (the samples were provided by the Sechenov First Moscow State Medical University). The liquid samples were certified based on the results of toxicology screening (TS)5, and were also previously characterized in the NARK-PHOSPHAN multiplex test [15].
To prepare dry samples, test strips (blanks) of filter paper (WHATMAN 903, USA) 0.45 cm wide and at least 3–4 cm long were used; the strips were soaked in urine by immersing them in a container with a liquid sample for 1 min, followed by air drying. The blanks were stored at a temperature of +4°C with a desiccant in a foil bag. The assay was performed using 0.45×0.45 cm fragments of a paper test strip containing dry material equivalent to 8 ± 0.4 μL of liquid urine. WHATMAN 903 paper ensured a standard sample volume per unit area and good preservation of the biomaterial.
Dry urine samples (31 samples in total) were prepared from 50 liquid samples as follows: 30 dry samples were prepared from 30 liquid samples contained ND in different combinations and concentrations; one sample (a negative dry sample) was prepared from a mixture of 20 liquid urine samples that contained various common medications but no drug compounds [15].
To study the stability of ND in dry samples during storage, the prepared samples were kept at temperatures of 25 ± 5°C, 4–8°C, or –20°C for 14 days, 6 months, or 12 months, respectively. The effect of biosample storage temperature was considered insignificant provided that the difference in the ND level before and after sample storage was less than 15%.
A modified version of the NARK-PHOSPHAN test system developed by us earlier [15] was used to analyze dry urine samples. Immunochips were eight test microarrays printed on the well bottom of a 96-well polystyrene plate (NUNC, Denmark) to detect the studied ND (BZE (cocaine); THC (cannabinoids); AMP (amphetamine); MOR (opiates); BZD (benzodiazepines); BAR (barbiturates); mAMP (methamphetamine); MTD (methadone), and one control zone C. The test zones sorbed the related ND-protein (serum albumin) conjugates, and the control zone sorbed the biotin-protein (serum albumin) conjugate (Fig. 1). The immunochips were printed using the technique of non-contact printing, which reduced the microarray variability and reduced the sorbable immunoreagent volume to 2.5 nL compared to the previous test system [15].
The created immunoassay chip allows eight ND (MOR, BZE, THC, MTD, BZD, BAR, AMP and mAMP) to be detected in one plate’s well. This assay is based on a competitive reaction between test sample ND and ND conjugates in the immunochip test zones for binding with biotinylated ND antibodies. In the absence of ND in the sample, specific biotin-labeled MAbs bind to the related microarrays in the plate wells, which are then detected using streptavidin labeled with the long-lived luminescent marker Pt-coproporphyrin. In the presence of ND in the sample, a portion of the specific biotinylated antibodies binds to ND and is removed during washing of the plate wells. The higher the ND concentration in the sample, the lower the residual signal level in the test microarray.
The layout of immunoassay drug test using paper test strips and pooled samples is presented in Fig. 2.
Square fragments measuring 0.45×0.45 cm were cut off test strips impregnated with urine samples along the perforation line and placed in the wells of an auxiliary non-absorbing flat-bottomed plate. An extracting solution (the solution volume varied 25–500 μL) containing a mixture of mouse biotinylated MAbs was added to each well, and the samples were incubated for 30 min. Then, 25 μL of the resulting reaction mixture was transferred into the plate wells with printed immunochips and incubated for 1 h. After washing, 25 μL of streptavidin-Pt-coproporphyrin conjugate was added to the wells and incubated for 15 min. The MAb and conjugate dilutions were prepared in a buffer (pH 7.75) solution containing 12.1 mg/mL of tris-(hydroxymethyl)-aminomethane, 0.1 mL/L of Tween 20, 0.5 mg/mL of BSA (bovine serum albumin), 8.7 mg/mL of sodium chloride, 0.5 mg/mL of sodium azide (all reagents from SIGMA, USA). All incubations were carried out upon shaking at 700 rpm at room temperature. The plate was then washed and dried.
The ND presence in the sample was assessed by the inhibition rate of antibody binding in the related test zone using the B/B0 ratio (B is the level of the phosphorescent signal at a given ND concentration, and B0 is the level of the phosphorescent signal at zero ND concentration), which characterizes the inhibition rate of the phosphorescence signal in the related test zone of the immunoassay. The performance of the procedure was monitored by the signal level detected in the control zone of the immunochip, where the streptavidin conjugate binds to the Pt-coproporphyrin luminescent label.
The phosphorescent signal was recorded using an IFI-05 photoluminescence pulse indicator (Immunoscreen, Russia) by scanning the bottom of the plate well with light pulses with an excitation wavelength of 365 nm in stroboscopic mode with a pulse repetition frequency of 10 kHz and the selection of the long-lived luminescence signal of Pt-coproporphyrin with a maximum of 645 nm and a decay time constant of 40 μs. The results were processed and presented using the indicator software. For each test sample and detected ND, the ratio (B/B0) of the phosphorescence signal intensities recorded in the related immunochip test zone in the wells with the test sample (B) and without the addition of the B0 sample was automatically calculated.
Measurements were carried out in triplicate with calculation of the mean value (M) of the measurement results and the standard error of mean (SE). For statistical processing of the results, the standard Microsoft Office Professional Plus Excel 2013 (version 15.0.4727.1000, USA) software was used.
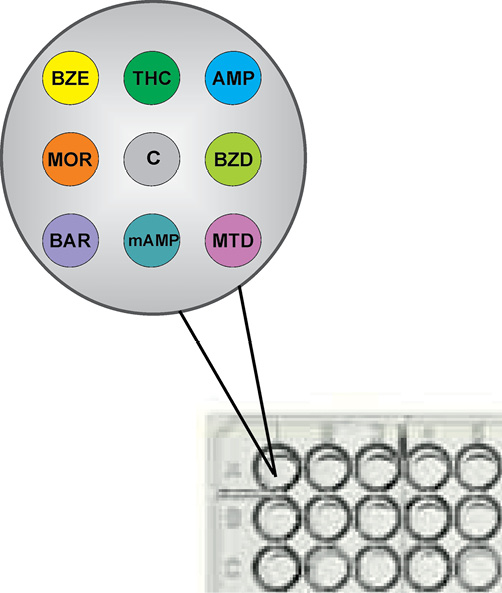
Figure prepared by the authors
Fig. 1. Layout of immunochip microarrays at the well bottom of 96-well microplates: BZE (cocaine); THC (cannabinoids); AMP (amphetamine); MOR (opiates); BZD (benzodiazepines); BAR (barbiturates); mAMP (methamphetamine); MTD (methadone); C (control)
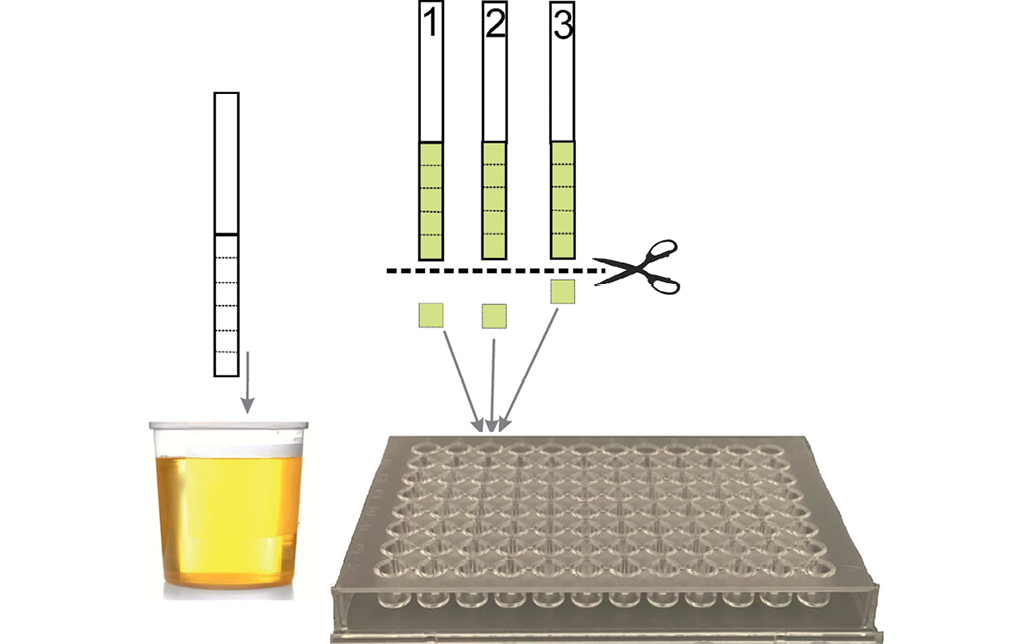
Figure was prepared by the authors
Fig. 2. Layout of urine drug test using paper test strips and the pooling procedure
Table. ND detection sensitivity in model extracts from urine samples dried on test strips using the PHOSPHAN technique
|
Narcotic drugs, psychotropic substances |
Analyte |
Proposed drug detection limits |
Estimated drug detection limit according to PHOSPHAN, ng/mL |
|
|
Screening |
Confirmation |
|||
|
Opiates |
MOR |
300 |
10 |
2 |
|
Cocaine |
BZE |
25 |
50 |
8 |
|
Cannabinoids |
THC |
15 |
15 |
6 |
|
Amphetamine |
AMP |
25 |
20 |
8 |
|
Methamphetamine |
MAMP |
25 |
20 |
6 |
|
Methadone |
MTD |
25 |
50 |
2 |
|
Benzodiazepines |
BZD |
20 |
50 |
4 |
|
Barbiturates |
BAR |
50 |
100 |
2 |
Table prepared by the authors based on their own data
RESULTS
When developing a multiplex immunoassay technology using pooled dried samples, the first step was to evaluate the detection sensitivity of each target protein. To that end, test strips were analyzed using urine samples with known levels of the target proteins. To prepare the model extracts, test strip fragments were placed in an extracting solution of varying volumes, ensuring that the target proteins concentration in the resulting reaction mixtures ranged 5–300 ng/mL. Based on the inhibition curves of the recorded signal for each of the eight ND (Fig. 3), the probable detection limits of the PHOSPHAN assay (as the ND levels corresponding to the recorded signal intensity value in the assay of a sample not containing this ND, minus two standard deviations) were calculated, which turned out to be 3–150 times lower for different NDs than the values recommended for toxicology screening tests (see Table).
Prior to examination of pooled samples, it was necessary to take into account that an increase in the number of simultaneously analyzed test strip fragments leads to an increase in the volume of the extracting solution required to fully saturate all the studied ND fragments, followed by the selection of 25 μL of the resulting reaction mixture for assay using immunochips. Our studies have shown that 50 μL of extracting solution is sufficient for analyzing a single fragment; for pools of 5, 10, 20, and 40 samples, the minimum volume of solution was 100, 160, 240, and 400 μL, respectively. Therefore, the urine sample dilution increased by a factor of 2, 3, 5, and 8, respectively, compared to the single sample assay.
Figure 4 shows an immunochip using results of testing of four dried human urine samples containing eight NDs in different combinations and concentrations; these samples were analyzed individually and as part of pools of 5, 10, 20, and 40 samples. Each pool contained one positive sample and a different number of strips of negative dried sample.
For all the samples studied, the B/B0 ratios in the test zones for the specific binding of drug compounds presented in the sample increased linearly from extremely low values to 75% along with an increase in the pooling frequency. The B/B0 ratios calculated in the test zones for the detection of drug compounds absent in the sample varied 70–110% (Fig. 4).
The conducted experiments (Fig. 4) allowed us to conclude that, in terms of analytical and economic parameters, pools of no more than 10 samples should be used. In this case, the B/B0 ratio recorded in the specific test zones of the immunochip in the presence of ND did not exceed 40% for all the samples studied. The ranges of the B/B0 parameter values in the test zones in the presence of ND did not overlap with the ranges of values recorded in the absence of ND in the sample. Based on the results obtained, the B/B0 value of 50% was selected as the threshold level for detecting ND.
Using the selected assay procedure based on 10-fold pooling (1 sample with ND + 9 samples without ND), we examined 30 dry samples containing initial liquid urine samples in various combinations of ND with the concentration ranges of 168–230 ng/mL BZE, 81–206 ng/mL THC, 405–1250 ng/mL AMP, 35–2500 ng/mL MOR, 251–800 ng/mL BZD, 75–350 ng/mL BAR, 115–471 ng/mL mAMP and 84–375 ng/mL MTD. The results are presented in Fig. 5, where the analysis data for samples containing and not containing the corresponding ND are shown separately for each test zone.
According to the data obtained (Fig. 5), the following substances were detected in the studied pools: cocaine (2 samples), cannabinoids (11 samples), amphetamines (6 samples), opiates (9 samples), benzodiazepines (7 samples), barbiturates (10 samples), methamphetamine (7 samples), and methadone (6 samples). Some samples contained both cocaine and methadone, benzodiazepines and barbiturates, as well as other ND mixtures in the presence of very high concentrations of opiates (> 2500 ng/mL) and amphetamines (> 1250 ng/mL).
For all positive dry samples containing ND, the B/B0 values in the related specific test zones were significantly lower than 50%. For the remaining samples containing other ND, the values were significantly higher than 50% (Fig. 5). Therefore, the developed multiplex phosphorescence immunoassay technique correctly detected the target ND in pools of 10 dry urine samples without any false-positive results with other ND.
The immunoassay specificity was also evaluated based on the study results of three pools composed of 10 test strip fragments from a negative urine sample. This sample was prepared from a mixture of liquid samples from patients whose urine contained various non-narcotic medications, including those that can cause false-positive reactions in the immunoassay (e.g., carbamazepine, amitriptyline, dextromethorphan, verapamil, etc.) [17]. During testing, the target NDs were not detected in any of the pools studied, which confirmed the specificity of the developed multiplex phosphorescence immunoassay technique.
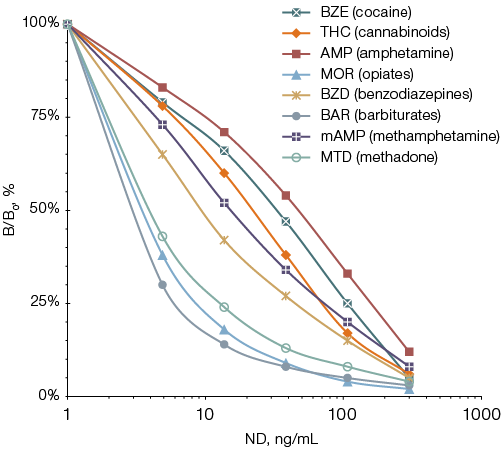
Figure prepared by the authors
Fig. 3. Inhibition curves of luminescent signals when eight NDs are detected in model extracts from urine samples dried on test strips: the data are presented in the form of the mean value (M) of the measurement results (n = 3); the standard error of the mean (SE) did not exceed 15%
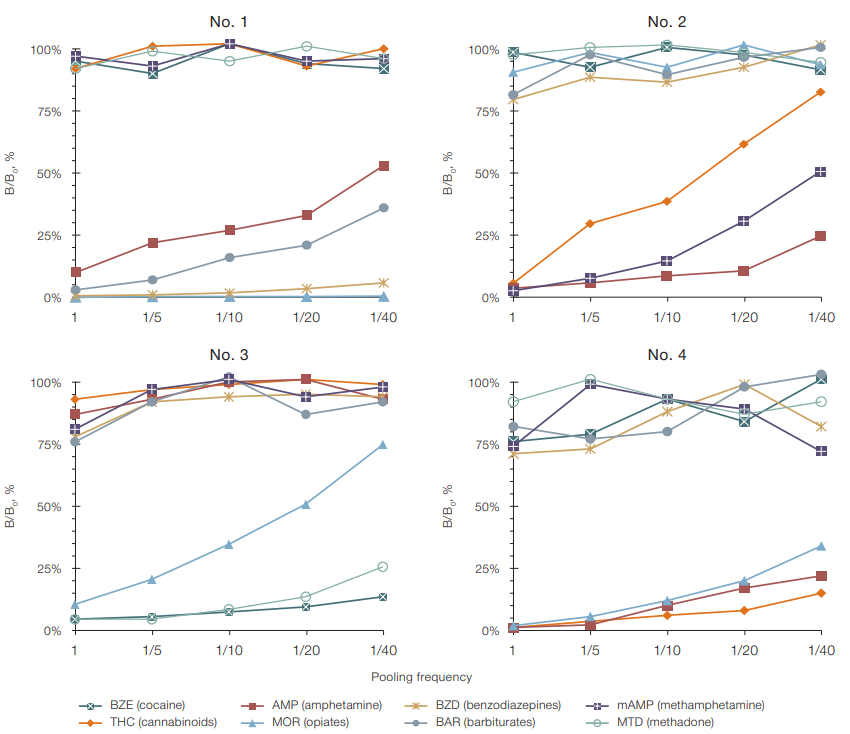
Figure prepared by the authors
Fig. 4. Phosphorescence signal inhibition rate when detecting eight NDs in dry urine samples (No. 1–4) using the PHOSPHAN technique, based on pooling frequency: ND baseline level in liquid urine samples (before drying on a paper test strip): sample No. 1 — 405 ng/mL AMP, 2500 ng/mL MOR, 506 ng/mL BZD and 75 ng/mL BAR; sample No. 2 — 115 ng/mL THC, 1250 ng/mL AMP and 471 ng/mL mAMP; sample No. 3 — 230 ng/mL BZE, 36 ng/mL MOR and 375 ng/mL MTD; sample No. 4 — 206 ng/mL THC, 1250 ng/mL AMP and 2500 ng/mL MOR; the data are presented in the form of the mean value (M) of the measurement results (n = 3); the standard error of the mean (SE) did not exceed 15%
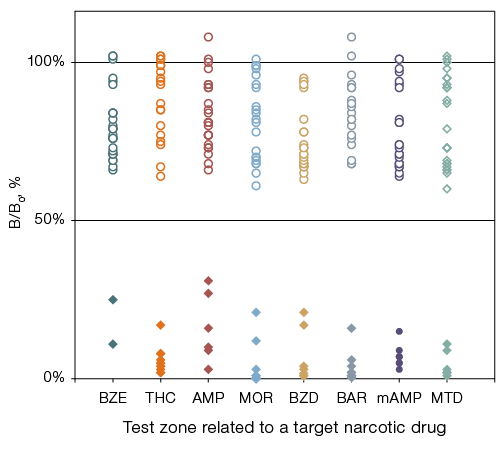
Figure prepared by the authors
Fig. 5. Results of detecting eight NDs in 30 dry urine samples examined as pools of 10 samples using the multiplex PHOSPHAN technique on microplate immunochips: BZE (cocaine); THC (cannabinoids); AMP (amphetamine); MOR (opiates); BZD (benzodiazepines); BAR (barbiturates); mAMP (methamphetamine); MTD (methadone); rhomb — samples contained ND, circle — samples non-contained ND, corresponding to this test area; the ordinate axis shows the average values for three repeated measurements
DISCUSSION
When developing a technological platform for multiplex immunoassay using pooled dry urine samples, we aimed to ensure its sensitivity and specificity comparable to those of highly sensitive reference methods of gas chromatography–mass spectrometry [12–14]. The development was based on the technology of multiplex phosphorescent immunoassay on microplate immunochips, which had previously demonstrated the possibility of achieving low detection limits for ND in liquid urine samples (sensitivity was 1 ng/mL for morphine and methadone, 0.5 ng/mL — for barbiturates, 2 ng/mL — for benzoylecgonine, methamphetamine, cannabinoids and benzodiazepines, 8 ng/mL — for amphetamine) in the absence of significant cross-reactions [15].
The study objective was to modify the PHOSPHAN technology for the assay of pooled dry samples. It is known that the assay of such samples is accompanied by an inevitable decrease in sensitivity due to incomplete desorption and additional dilution during the extraction of analytes from the paper. To address this issue, we employed a series of technological approaches to enhance the sensitivity of the modified PHOSPHAN tests while maintaining their specificity.
The immunochip formation was carried out using a non-contact printing method with a microarray volume of 2.5 nL (instead of 20–25 nL in the previously used contact printing method) [15]. This reduction in the amount of conjugated ND antigen in the test microzone of the immunochip resulted in a 2–3-fold decrease in the ND detection limit in competitive immunoassay.
The method includes a step of ND pre-extraction from paper test strips (examined as fragments of 4.5×4.5 mm in size) in the presence of specific biotinylated antibodies. The pre-interaction of the ND sample with antibodies in the absence of competition with the BSA conjugate for the ND provided a greater inhibition rate and, accordingly, an additional reduction in the detection threshold.
The method of preparing dry samples by immersing the test strip in a container with a human urine sample ensures soaking of a sufficiently large area, which allows for up to five repeated measurements of the sample (Fig. 2), including for confirmation testing in the event of ND detection in the pool examined. The dimensions of the analyzed test strip fragments (4.5 x 4.5 mm) allow for the pooling of up to 10 samples in a single microplate well.
The proposed method for ND assay from a pool of dry urine samples can form the basis for a new screening technique that involves collecting urine samples, their application onto paper test forms, and transportation to a laboratory, including by mail, for ND testing.
It is important to note that there are no special requirements for the “cold chain” when shipping dry samples, as the ND level in paper-dried urine samples remains relatively stable. According to our research, the levels of eight analytes under study decreased by no more than 15% during storage for five days at room temperature, one month at 4°C, and up to six months in frozen form. This is consistent with the findings of other researchers regarding a wide range of analytes detected in paper-dried clinical urine and blood samples [14].
In the laboratory, the collected samples can be combined into pools of 10 samples and subjected to a preliminary assay, where each pool is tested in a single well of a microplate immunoassay. In cases where the pool produces a positive result for one of the target ND, a confirmation test is performed, which involves testing each of the 10 samples in the pool and determining the exact concentration of the ND in the sample using the corresponding calibration curves provided in the biochip analyzer software.
On the basis of the results obtained, we set the threshold level for recognizing a test result as positive to B/B0 = 50%. This criterion should be defined more precisely by conducting research using a wider sampling. It may be necessary to select different evaluation criteria for each analyzed ND in order to reduce the likelihood of obtaining false-positive or false-negative results.
Due to its high sensitivity, the developed method of multiplex phosphorescence immunoassay allowed the detection of eight main NDs in a pool of 10 samples. The specificity of the method was confirmed by the correctness of ND identification in all the studied samples, including those containing other NDs, and the absence of non-specific reactions when analyzing a pool of negative urine samples containing various non-narcotic medications.
The proposed technological platform has no fundamental limitations for extending the panel of NDs detected in a single immunoassay. Our preliminary experiments have shown that it is possible to additionally include synthetic cannabinoids (K2) and cathinones (MDPV), phencyclidine, fentanyl, and ecstasy (MDMA) in the detected ND list, since their sensitivity and specificity meet the requirements for test development using the sample pooling technique. Thus, the number of simultaneously detectable substances can be extended to at least 13, covering all groups of substances that must be monitored during chemical and toxicological examinations of drug-dependent individuals.
CONCLUSIONS
- A multiplex phosphorescence microplate immunoassay has been developed for detecting eight main groups of narcotic and psychotropic substances in pools of paper-dried urine samples.
- The detection limits of the target ND in extracts from urine samples dried on test strips ranged 2–8 ng/mL, which is significantly lower than the detection limits recommended for screening examinations.
- The proposed approach to multiplex immunoassay of NDs in pools of dry urine samples can form the basis of a new screening technique that includes urine sampling, application to paper test forms, and transportation to a laboratory for ND testing.
- According to our estimates, the use of multiplex phosphorescence immunoassay and pooled urine samples will significantly (by more than 10 times) reduce the cost of testing compared to conventional immunochromatographic assay technologies. A centralized laboratory equipped with a high-performance domestic photoluminescence indicator of the IFI-05 series, can process at least 10,000 samples per work shift, divided into pools of 10 samples. During mass examinations of groups of people at critically important industrial facilities, the cost of one examination for 8–13 NDs will not exceed 20–30 rubles, or 2–3 rubles per one type of ND detected.
- The work conducted by specialists of the State Scientific Research Institute of Biological Engineering and the Immunoscreen company is aimed at further development of highly cost-effective domestic technologies for ND screening using pooled urine samples and conducting pilot surveys, thus contributing to organization of improved dispensary drug control in the entitled territories of the Federal Medical and Biological Agency (FMBA) of Russia.
1 United Nations Office on Drugs and Crime (UNODC). World Drug Report; 2024.
2 Roberts L. Drug addiction in Russia: statistics, therapy, prevention. Modern-info.com. 2025.
3 Federal Law 61-FZ On Medicine Circulation. Moscow.: Federation Council; 12.04.2010.
4 WADA. Technical Document Dried Blood Spots (DBS) for Doping Control. 2023.
5 Methodical Guidelines “Rules for conducting chemical and toxicological tests for narcotic drugs, psychotropic substances, and other toxic substances (their metabolites) in the human body during medical examinations and medical assessments of certain citizens categories”. Moscow.: Ministry of Health of the Russian Federation; 2015.
References
1. Korshunov VA, Mindlina AYa, Viazovichenko YuE. Analysis of the Russian primary drug abuse prevention system and proposals for its optimization. Sechenovski Vestnik. 2016;1(23):31–8 (In Russ.).
2. Pozdniakova ME. The drug situation in Russia and new models of drug abuse. Sociology of Medicine. 2016;15(1):25–30 (In Russ.).
3. Kaimak YeV. The threat of illicit drug use at critically important and potentially dangerous objects of national infrastructure in Russia. Biosphere. 2012;2:107–15 (In Russ.).
4. Grobe N, Cherif A, Wan X, Don Z, Kotanko P. Sample pooling: burden or solution? Clinical Microbiology and Infection. 2021;27:1212–20. https://doi.org/10.1016/j.cmi.2021.04.007
5. Mardal M, Kinyua J, Ramin P, Miserez B, van Nuijs ALN, Covaci A, et al. Screening for illicit drugs in pooled human urine and urinated soil samples and studies on the stability of urinary excretion products of cocaine, MDMA, and MDEA in wastewater by hyphenated mass spectrometry techniques. Drug Testing and Analysis 2017;9(1):106–14. https://doi.org/10.1002/dta.1957
6. Dziadosz M, Klintschar M, Teske J. Sample pooling as an effective way of simultaneous analysis of new designer drugs together with synthetic cannabinoids in human serum provided by therapy and forensic psychiatric centres. Medicine, Science and the Law. 2016;56(2):155–6. https://doi.org/10.1177/0025802415587319
7. Olkhovskiy IA, Gushchin VA, Kuznetsova NA, Rubalsky OV. Using the samples pools in SARS-COV-2 RNA virus testing by polymerase chain reaction. Laboratory Service. 2021;10(1):68–75 (In Russ.). https://doi.org/10.17116/labs20211001168
8. Phan T, Tran NYK, Gottlieb T, Siarakas S, McKew G. Evaluation of the influenza and respiratory syncytial virus (RSV) targets in the AusDiagnostics SARS-CoV-2, Influenza and RSV 8-well assay: sample pooling increases testing throughput. Pathology.2022;54(4):466–71. https://doi.org/10.1016/j.pathol.2022.02.002
9. Mulu A, Alemayehu DH, Alemu F, Tefera DA, Wolde S, Aseffa G, et al. Evaluation of sample pooling for screening of SARS CoV-2. PLoS One. 2021;16(2):e0247767. https://doi.org/10.1371/journal.pone.0247767
10. Griesemer SB, Van Slyke G, St George K. Assessment of sample pooling for clinical SARS-CoV-2 testing. Journal of Clinical Microbiology. 2021;59(4):e01261–20. https://doi.org/10.1128/JCM.01261-20
11. Tan JG, Omar A, Lee W, Wong MS. Considerations for group testing: a practical approach for the clinical laboratory. The Clinical Biochemist. Review. 2020;41(3):79–92. https://doi.org/10.33176/AACB-20-00007
12. Meikopoulos T, Gika H, Theodoridis G, Begou O. Detection of 26 Drugs of Abuse and Metabolites in Quantitative Dried Blood Spots by Liquid Chromatography-Mass Spectrometry. Molecules. 2024;29(5):975. https://doi.org/10.3390/molecules29050975
13. Mazzarino M, Di Costanzo L, Comunità F, Stacchini C, de la Torre X, Botrè F. UHPLC-HRMS method for the simultaneous screening of 235 drugs in capillary blood for doping control purpose: Comparative evaluation of volumetric and non-volumetric dried blood spotting devices. ACS Omega. 2022;7:31845–68. https://doi.org/10.1021/acsomega.2c01417
14. Gaugler S, Al-Mazroua MK, Issa SY, Rykl J, Grill M, Qanair A, et al. Fully automated forensic routine dried blood spot screening for workplace testing. Journal of Analytical Toxicology. 2019;43(3):212–20. https://doi.org/10.1093/jat/bky074
15. Bekman NI, Pomelova VG, Osin NS. Multiplex analysis of drugs of abuse by the use of PHOSPHAN-based immunochip technology. Russian Clinical Laboratory Diagnostics. 2018;63(3):178–83 (In Russ.). EDN: YVQOVH
16. Bekman NI, Pomelova VG, Osin NS. New screening methodology for identifying drug addicts. Interpretation of laboratory test results: Proceedings of the XXIX All-Russian scientific and practical conference with international participation. Moscow; 2024 (In Russ.).
17. Saitman A, Hyung-Doo P, Fitzgerald R. False-positive interferences of common urine drug screen immunoassays. Journal of Analytical Toxicology. 2014;38(7):387–96. https://doi.org/10.1093/jat/bku075
18.
About the Authors
N. I. BekmanRussian Federation
Natalia I. Bekman, Cand. Sci. (Chem.)
Moscow
Dubna, Moscow Region
V. G. Pomelova
Russian Federation
Vera G. Pomelova, Dr. Sci. (Biol.)
Moscow
Dubna, Moscow Region
N. S. Osin
Russian Federation
Nikolai S. Osin, Dr. Sci. (Biol.)
Moscow
Dubna, Moscow Region
Supplementary files
Review
For citations:
Bekman N.I., Pomelova V.G., Osin N.S. Integration prospects for the multiplex phosphorescence immunoassay of pooled dry urine samples into screening examinations in dispensary drug control. Extreme Medicine. 2025;27(3):400-409. https://doi.org/10.47183/mes.2025-261

















