Scroll to:
Comparative assessment of proteomic regulation of bone tissue during 21-day head-down bed rest (–6°) and 21-day dry immersion
https://doi.org/10.47183/mes.2025-296
Abstract
Introduction. Experimental possibilities during actual spaceflight are limited, making ground-based models, such as dry immersion (DI) and head-down bed rest (HDBR) tests, highly relevant. Changes in bone tissue are induced by alterations in a complex set of environmental factors at the proteomic level, compensating for changes caused by reduced gravity and decreased motor activity. However, upon continued exposure, other regulatory circuits are activated.
Objective. Comparative assessment of proteomic regulation of bone tissue status in 21-day HDBR (tilted at 6°) and 21-day DI tests.
Materials and methods. Using mass spectrometry methods, plasma samples from 8 healthy male volunteer subjects (mean age 20– 44 years) under the conditions of 21-day HDBR and 10 subjects (mean age 23–34 years) under 21-day DI were studied. The Perseus software was used for statistical analysis and identification of molecular functions and biological processes involving the proteins. The correspondence of major biological processes, according to gene ontologies specified in the GO database, and identified proteins was established using the knowledge base of the ANDSystem and STRING.
Results. Nine proteins with significantly altered levels on Day 21 of HDBR (p < 0.05) and eight proteins with significantly altered levels on Day 21 of DI (p < 0.05) were identified. These proteins are associated with biological processes occurring in bone tissue. Some of the identi© Л.Х. Пастушкова, А.Г. Гончарова, Д.Н. Каширина, И.М. Ларина, 2025 fied proteins form stable protein–protein interaction (PPI) networks, indicating potential co-expression. Two common proteins — haptoglobin (Hp) and glutathione peroxidase (GPx) — were identified on Day 21 of both DI and HDBR.
Conclusions. The findings offer an insight into the proteomic mechanisms regulating biological processes in bone tissue of healthy individuals under the influence of 21-day HDBR and 21-day DI. Annotations for each protein involved in bone tissue biological processes during 21-day HDBR (tilted at 6°) and 21-day DI are provided. These results are of great importance for aerospace and clinical medicine.
For citations:
Pastushkova L.Kh., Goncharova A.G., Kashirina D.N., Larina I.M. Comparative assessment of proteomic regulation of bone tissue during 21-day head-down bed rest (–6°) and 21-day dry immersion. Extreme Medicine. 2025;27(4):558-568. https://doi.org/10.47183/mes.2025-296
INTRODUCTION
The knowledge of biological changes in bone tissue induced by prolonged exposure to microgravity, as a component of the set of spaceflight (SF) factors, is of paramount importance for space agencies planning to conduct deep space exploration missions.
Weightlessness causes physiological changes affecting the musculoskeletal system and interrelated sensory, neuromuscular, vascular, and other processes. In view of the limited experimental opportunities during actual spaceflight, ground-based models [1] can be used to assess the effects of microgravity, to identify gravitational mechanisms regulating the body’s physiological systems, and to elucidate adaptation mechanisms to weightlessness [1].
Comparisons of the results obtained by ground-based model experiments (head-down bed rest, HDBR; dry immersion, DI) and those during actual SF have demonstrated the utility of ground-based simulations of specific SF factors for studying fundamental patterns and changes in the organism. The use of these models has become essential for research purposes, allowing for a broader participant pool and enhanced scientific significance through the analysis of various protocols (e.g., different durations or use of countermeasures) [2]. Notably, the use of invasive procedures (e.g., venipuncture for blood sampling, biopsies) and the logistics of cargo delivery to the orbit and back to the Earth present significant challenges [2], further emphasizing the importance of ground-based model studies.
Extended knowledge in this field is crucial for the social and economic aspects of maintaining the health, work capacity, and social activity of astronauts. Research into the effects of physical inactivity and forced bed rest on human health is highly relevant in the context of modern health issues and population aging [2]. Reduced mechanical loading on the skeleton, caused by bed rest and/or spaceflight, leads to bone mass loss, reflected in a decrease in the bone mineral density (BMD) both throughout the entire skeleton and in specific sites, such as the spine, femoral neck, and tibia [3, 4].
Baran et al. and Man et al. identified structural changes in the radius and tibia using peripheral quantitative computed tomography (pQCT) and high-resolution peripheral quantitative computed tomography (HR-pQCT) under spaceflight conditions [5][6].
Alterations in hormonal status accompanying bone resorption and formation processes have been noted in several studies. For instance, disrupted circadian rhythms of somatotropin and a decrease in its average daily concentration during a 370-day HDBR experiment were described [7]. Grigoriev et al. and Austermann et al. demonstrated changes in the levels of hormones regulating calcium metabolism during both 120-day and 370-day HDBR. Specifically, by day 75 of the experiment, parathyroid hormone (PTH) levels were below baseline, while the calcitonin (CT) concentration was elevated [8][9]. Inoue et al. established that insulin-like growth factor I (IGF-I), its binding protein (IGFBP), and insulin-like growth factor binding protein 3 (IGFBP-3) increased during 120-day bed rest, suggesting potential IGF-I resistance in bones under reduced mechanical load and strain [10].
Although model studies cannot fully replicate the conditions experienced by astronauts during SF, there is a notable similarity in the pathophysiological changes observed during prolonged mobility restriction (bed rest) and the issues faced by astronauts [2].
In DI tests, the lack of mechanical support for specific body areas during immersion creates a state akin to weightlessness, termed unloading, which induces physiological changes in the musculoskeletal and other bodily systems [1]. Hypokinesia and hypodynamia are primary characteristics of physical inactivity induced by DI. Hypodynamia implies reduced postural muscle load, while hypokinesia represents a decrease in motor activity. In addition to the acute restriction of normal muscle activity and reduced load on muscles and bones, thermoneutral immersion rapidly induces a significant decline in muscle tone and tension [11][12], which is unattainable even with prolonged bed rest models.
Kotov et al. found that after seven days of DI, the bone mineral density (BMD) in the lower skeleton (proximal femoral epiphysis) decreased by 2%, while the density in the upper body (skull, hand, rib bones) was approximately 2% higher than the baseline values. Moreover, three weeks of recovery after DI were sufficient to reverse these bone density changes [13]. It is hypothesized that these changes are a secondary effect of the cranial fluid shift to the upper body, where increased hydrostatic pressure promotes the movement of ions and proteins into the bone. Thus, DI appeared to exert a similar effect as HDBR on bone resorption in specific body regions. Baecker et al. showed that markers of bone resorption increased as early as day 2 of bed rest [14]. Kotov et al. also confirmed the rapid onset of bone tissue degradation under immobilization conditions, such as DI or HDBR.
According to Markin et al., biochemical processes involved in bone formation were not affected by day 7 of DI, as evidenced by the lack of changes in serum alkaline phosphatase (ALP) concentration [13][15], serum procollagen type I N-terminal propeptide (PINP), and bone-specific alkaline phosphatase (BAP). Markers of bone resorption, such as tartrate-resistant acid phosphatase (TRAP) and urinary C-terminal telopeptide of type I collagen (CTX), showed a slight increase during a 7-day DI [16]. However, Markin et al. did not detect any changes in the activity of total acid phosphatase as a biomarker of osteoclast activity during a 7-day DI [15].
It is evident that alterations in bone tissue are induced by a complex set of environmental factors at the proteomic level, initially compensating for reduced gravity and decreased motor activity. However, upon prolonged exposure, additional regulatory pathways become engaged.
In this research, we aim to carry out a comparative assessment of proteomic regulation of bone tissue status under the conditions of 21-day head-down bed rest (tilted at –6°) and 21-day dry immersion.
MATERIALS AND METHODS
The 21-day head-down bed rest (HDBR) study involved eight healthy male volunteers aged 20–44 years. The participants were maintained at a –6° head-down tilt position relative to the horizontal for 21 days under controlled conditions at the MEDES Research Center as part of the joint Russian-French CaDy WEC laboratory program (Toulouse, France, 2014). No countermeasures to prevent adaptive physiological changes were implemented. The participants received a standardized diet with controlled nutrient content and monitored water intake. Blood samples were collected prior to the study (baseline) and on day 21 of HDBR.
The 21-day dry immersion (DI) study involved 10 healthy male volunteers aged 23–34 years, approved by the IBMP RAS Medical Expert Commission. All participants provided their written informed consent (IBMP RAS Bioethics Committee Protocol No. 483, 03.08.2018). The study complied with the requirements of Helsinki Declaration for participant safety and risk management. The experiment was conducted at IBMP RAS using the Dry Immersion facility, part of the “Medical and Technical Complex for Innovative Space Biomedicine Technologies” research facility (RSF grant No. 19-15-00435). Proteomic research was supported by state assignment No. FMFR-2024-0032. Both studies used comparable diets and hydration protocols.
Plasma samples were collected at identical timepoints in both studies, including seven days prior to exposure (baseline) and on day 21 of DI/HDBR. Blood was drawn from the cubital vein (5 mL) into EDTA tubes after fasting. Samples were centrifuged in 9 mL K3 EDTA vacuum tubes at 3000 rpm (MPW-350R centrifuge, Poland) for 10 min at 4°C. The preparation technique and the subsequent chromatography-mass spectrometry analysis were identical for all biological samples, regardless of the experimental exposure factor, in order to ensure the validity of the comparison of the results obtained in both experiments.
FASP filters were used for sample preparation. Mass spectrometry analysis was performed using a MaXis 4G spectrometer (Bruker Daltonics, Germany) equipped with the MaxQuant software. Peak lists included up to eight major peaks per 100 Da window. The SwissProt database (forward/reverse) with 10 ppm precursor mass tolerance was used for identification purposes. Peptides were identified with ≥7 amino acids, FDR (false discovery rate) 0.01, and the “match between runs” option.
Statistical analysis was conducted using the Perseus software with Mann–Whitney U tests for small samples [17]. Functional annotation was carried out using the ANDSystem and STRING databases with GO term enrichment1.
RESULTS AND DISCUSSION
The results of a comparative proteomic analysis of statistically significant differentially expressed proteins in ground-based model studies is presented in the Table.
The analysis of the data presented in the Table reveals statistically significant differences (p < 0.05) in protein levels compared to the baseline values under 21-day HDBR conditions. These include increased levels of APOE, Hp, complement C5 alpha chain, GPx3, HCII, and decreased levels of CD146 antigen, AngII, and CHLE. Meanwhile, under 21-day DI conditions, statistically significant increases were observed in PHLD, PON1, TTHY, TRFE, VTNC, as well as Hp and GPx3 compared to the baseline values.
Angiotensinogen (AGT gene) is involved in bone remodeling regulation. The renin-angiotensin-aldosterone system (RAAS) is known to participate in bone tissue regulation. Angiotensin II activates osteoclasts through increased expression of receptor activator of nuclear factor κ-B ligand (RANKL) on osteoblasts, leading to reduced BMD. The use of angiotensin II receptor blockers is associated with lower rates of bone fractures [18]. Some evidence suggests that RAAS blockade may reduce the risk of osteoporotic fractures [19]. However, other studies indicate that RAAS blockers do not reduce and may even increase the incidence of osteoporotic fractures [20]. RAAS components are expressed in bone tissue, activating local RAAS responses that lead to decreased bone density [21]. Angiotensinogen increased interleukin-6 secretion in vitro and reduced alkaline phosphatase activity only in otosclerotic cells. These observations suggest a connection between local renin-angiotensin system activity and otosclerosis, suggesting new therapeutic possibilities.
Mineralocorticoid receptors have also been identified in human osteoblasts, osteoclasts, and bone cells. Previous research indicates that local bone RAAS plays an important role in various causes of osteoporosis. RAAS blockers may reduce BMD loss through cascades involving angiotensin II type 1 receptor (AT1R), the ligand-receptor system (OPG/RANKL), and angiotensin-converting enzyme 2 (ACE2/Ang)(1-7)/Mas. By restoring bone physicochemical properties and reducing fracture risk, RAAS blockers could serve as an effective adjuvant therapy for osteoporosis [21].
Heparin cofactor II HCII (SERPIND1 gene) stimulates osteogenic activity. Studies on HCII effects have investigated bone formation models stimulated by human tumors. HCII induced new bone growth over the cranial surface, even at a distance from the tumor mass. This suggests bone growth induction through growth factor production and the combined action of multiple factors on bone tissue [22].
Cholinesterase (BCHE gene) is an esterase with a broad substrate specificity. Acetylcholinesterase inhibitors (AChEIs) are known to stimulate acetylcholine receptors and are used in the treatment of Alzheimer’s disease, providing protection against osteoporosis and inhibiting osteoclast differentiation and function. AChEIs variably reduced RANKL-induced transcription of nuclear factor of activated T cells 1 (Nfatc1) and osteoclast marker gene expression (primarily donepezil and rivastigmine, rather than galantamine). Additionally, AChEIs differentially inhibited RANKL-induced MAPK signaling, accompanied by reduced acetylcholinesterase transcription. Finally, AChEIs protected against OVX-induced bone loss primarily by inhibiting osteoclast activity. Collectively, AChEIs (mainly donepezil and rivastigmine) positively affected bone protection by suppressing osteoclast function through MAPK and Nfatc1 signaling pathways via downregulation of acetylcholinesterase [23].
Apolipoprotein E (APOE gene) is a biomarker of fracture risk and an indicator of lower BMD in patients with osteoporosis. The APOE2 and APOE4 alleles have been associated with lower BMD, as well as with higher levels of serum C-terminal telopeptide and urinary deoxypyridinoline, which are biomarkers of bone resorption. Codominance of the APOE3 allele was also associated with fewer bone fractures in these patients over a 5-year follow-up period [24]. Apolipoprotein E levels were shown to increase significantly on day 7 of a space flight [25].
During the study under HDBR conditions, 33 significantly altered proteins were identified. Nine of these are associated with biological processes occurring in bone tissue (Fig. 1) and showed statistically significant changes compared to the baseline values. The proteins AGT and AGT II are annotated together as they are local regulators of bone tissue, belonging to the same group of angiotensin system regulators. Consequently, they are bioinformatically grouped by the ANDVisio software.
The results of 21-day HDBR exposure revealed statistically significant associations between specific proteins and key bone tissue biological processes. The ossification process demonstrated connections with four proteins, i.e., MUC18, GPX3, AngII, and APOE. Osteoblast differentiation was associated with one protein (Complement C5 alpha chain), while bone mineralization correlated with AngII levels. Bone development involved two proteins — AngII and CHLE, and bone resorption was linked to two proteins — AngII and Hp. Osteoclast differentiation similarly involved two proteins (AngII, Hp), bone growth was associated with one protein (HCII), and bone remodeling regulation correlated with AngII.
Our study also identified protein–protein interaction networks related to bone tissue regulation under 21-day HDBR conditions, with the corresponding data presented in Fig. 2. Six proteins — apolipoprotein E, haptoglobin, complement C5 alpha chain, angiotensin, cholinesterase, and extracellular glutathione peroxidase — formed an interconnected protein–protein interaction network suggesting potential co-expression. One protein (CD146 antigen) remained outside this network (Fig. 2). The constructed network reflects the mutual influence of these proteins on common bone tissue targets.
The protein interactions indicate coordinated regulation of bone metabolic pathways during mechanical unloading, with angiotensin II (AngII) emerging as a central regulator across multiple processes, including ossification, mineralization, and bone resorption. The network analysis provides insights into potential molecular mechanisms underlying HDBR-induced bone adaptation and suggests targets for countermeasure development.
Bergdolt et al. demonstrated that the complement protein C5 receptor (C5aR1) plays a crucial role in bone metabolism and fracture healing, being highly expressed on immune and bone cells, including osteoblasts and osteoclasts. C5aR1 induces osteoblast migration, cytokine production, and osteoclastogenesis. C5aR1 signaling in osteoblasts may potentially influence the RANKL/OPG signaling pathway balance that regulates bone tissue homeostasis, involving the receptor activator of nuclear factor κ-B ligand (RANKL) and osteoprotegerin (OPG), leading to increased bone resorption. Binding to the C5AR1 receptor triggers various responses, including intracellular calcium release, smooth muscle contraction, increased vascular permeability, and histamine release from mast cells and basophilic leukocytes [26]. Ignatius et al. suggested that complement may enhance the inflammatory response of osteoblasts and increase osteoclast formation, particularly in pro-inflammatory environments such as during bone healing or inflammatory bone diseases [27].
Pimenta-Lopes et al. established that genetic deletion of C5ar1, the receptor for the anaphylatoxin C5a, or treatment with a C5AR1 inhibitor reduced monocyte chemotaxis and osteoclast differentiation, partially preventing bone loss and osteoclastogenesis during chemotherapy or ovariectomy. Thus, inhibition of alternative complement pathways may have specific therapeutic effects in osteopenic disorders [28]. Meanwhile, Kunimatsu et al. showed that the cell surface glycoprotein MUC18 (MCAM gene) acts as a surface receptor that triggers tyrosine phosphorylation of FYN and a temporary increase in intracellular calcium concentration. This protein stimulates a cell population capable of bone formation and transendothelial migration in vivo, inducing bone tissue regeneration [29].
Thus, the identified changes indicate the involvement of both systemic and local protein regulators of bone tissue status in the biological processes of bone metabolism. Notably, the local bone RAAS plays an important role in the development of osteoporosis of various etiologies already in the early stages of exposure to the set of simulated SF factors. RAAS blockers may reduce BMD loss through AT1R, OPG/RANKL, ACE2/Ang (1-7)/Mas cascades.
In turn, AChEI inhibition exerts a positive effect on bone protection by suppressing osteoclast function through MAPK and Nfatc1 signaling pathways via downregulation of AChE. Changes in apolipoprotein E levels may reflect the activation of a protective biological process of osteogenesis in response to the duration of HDBR exposure.
An analysis of the blood plasma proteome on day 21 of DI identified 31 proteins with significantly altered levels, out of which eight proteins were associated with the regulation of biological processes in bone tissue, such as bone remodeling (serum paraoxonase), osteoclast differentiation (haptoglobin and transthyretin), bone mineralization (transthyretin), bone regeneration (transthyretin, fibronectin), osteoblast differentiation (fibronectin), resorption (transthyretin, serotransferrin), BMP-4 signaling pathway (fibronectin), and bone biosynthesis, including bone growth and development processes (fibronectin) (Fig. 3).
These proteins form a stable protein–protein interaction network (Fig. 4). The analysis of protein–protein interactions related to bone tissue regulation under 21-day DI conditions are presented in Fig. 4. Seven proteins, including haptoglobin, extracellular glutathione peroxidase, serum paraoxonase, serotransferrin, transthyretin, vitronectin, and fibronectin, form a protein–protein interaction network, indicating potential co-expression, while one protein (phosphatidylinositol-glycan-specific phosphatase) remains outside this network (Fig. 4). The constructed network reflects the mutual influence of these proteins on common bone tissue targets.
Fibronectin (FN1 gene) participates in osteoblast condensation through matrix assembly via fibronectin fibrillogenesis, regulates type I collagen deposition by osteoblasts, and acts as a ligand for the immunoglobulin-like receptor family membrane protein (LILRB4), inhibiting monocyte activation. Fibronectin fibrillogenesis is involved in bone mineralization processes. Specific regulation of FN1 during different phases of osteoblast differentiation has been established.
In studies by Xiong et al., fibronectin-1, thrombospondin-1, and biglycan were identified as key bone mineralization genes, with their upregulation associated with potential disturbances in bone remodeling processes. Fibronectin-1 (FN1), thrombospondin-1 (THBS1), and biglycan (BGN) were determined as the most significant genes in treating non-union fractures, highlighting the crucial role of FN1, THBS1, and BGN in extracellular matrix mineralization dynamics and bone regeneration [30]. Increased FN1 expression promotes fracture healing by activating the TGF-β/PI3K/Akt signaling pathway [31].
Serotransferrin (TF gene) is involved in regulating biological processes of bone resorption and osteoclast differentiation. Higher levels of soluble transferrin receptor (sTfR) correlate with a lower trabecular number, cortical thickness, and cortical pore diameter. The relationship between the tibial bone density and strength and low circulating concentrations of bone resorption and formation markers with serotransferrin levels likely results from the direct role of iron ions in collagen synthesis [32][33]. Serotransferrin levels in dry blood spot extracts from cosmonauts were significantly altered after three and six months of SF [25]. This indicates that ground-based model studies do reproduce some proteomic biological processes of bone tissue regulation observed at different time points during SF [11].
Vitronectin (VTN gene) is present throughout the mineralized bone matrix of cancellous and cortical bones, suggesting its participation in bone remodeling through bone formation, resorption, and osteogenesis biological processes. Vitronectin is known to interact with glycosaminoglycans and proteoglycans, inhibiting the membrane-damaging effect of the terminal cytolytic complement pathway. Vitronectin deficiency was shown to increase osteoclast numbers and reduce total femoral bone volume in an ovariectomized mouse osteoporosis model [34]. Our previous studies noted that vitronectin levels significantly decreased after six months of SF [25], confirming the role of vitronectin in regulating osteogenesis and bone remodeling under DI and SF conditions in overall bone volume formation.
Serum paraoxonase/arylesterase 1 (PON1 gene) plays an important role in maintaining the buffering colloidal properties of intervertebral discs. Low levels of PON1 expression were established as a predictor of nucleus pulposus degeneration in intervertebral discs. Inflammation and oxidative stress can deteriorate the cellular environment of the nucleus pulposus, leading to intervertebral disc degeneration. Paraoxonase is an enzyme with anti-inflammatory and antioxidant effects. Aydın et al. investigated PON1 expression in 88 human intervertebral disc samples and rat models, measuring tumor necrosis factor (TNF-α), interleukin (IL-1β), mitosuperoxide (SOX), aggrecan, and collagen II levels in nucleus pulposus cells. PON1 expression was significantly suppressed in degenerative human and rat intervertebral discs. PON1 levels were significantly reduced in degenerative cell models induced by TNF-α and oxidative stress (H2O2). TNF-α and interleukin-1β (IL-1β) levels clearly increased, while aggrecan and collagen expression decreased in cells transfected with PON1 siRNA. PON1 levels were also significantly higher in patients with osteoporotic hip fractures, particularly intertrochanteric femoral fractures and femoral neck fractures, compared to the control group [35].
Thus, low PON1 expression is a predictor of severe intervertebral disc dysfunction. PON1 plays a crucial role in maintaining the homeostatic balance of the intervertebral disc nucleus pulposus. Therapeutic approaches targeting PON1 may be beneficial for alleviating nucleus pulposus dysfunction in the future.
Phosphatidylinositol-glycan-specific phospholipase D (GPLD1 gene) primarily functions to hydrolyze the inositol phosphate bond in phosphatidylinositol glycan-anchored proteins, releasing these proteins from the membrane. Additionally, associations between the phosphatidylinositol-glycan-specific phospholipase locus and alkaline phosphatase levels were established, suggesting the specificity of this protein for bone tissue [36].
Transthyretin (TTR gene), TTHY is a transport protein involved in regulating biological processes of bone resorption and growth. Transthyretin levels gradually decrease with a reduction in BMD in osteoporosis patients [37]. Similar changes in the levels of this protein were identified in the dry blood spots of cosmonauts after six months of SF [25].
Two common proteins were identified regarding their participation in the biological processes of bone tissue regulation, observed both following 21 days of HDBR and 21 days of DI. These proteins are haptoglobin (Hp) and glutathione peroxidase (GPx3) (Fig. 5).
When examining the participation of these proteins in regulating the biological processes of bone tissue, the following observations should be mentioned. Thus, altered haptoglobin concentrations (Hp gene) were detected at day 21 in both DI and HDBR exposure conditions. Haptoglobin participates in regulating osteoclast differentiation and bone resorption. The protein Zscan10 is likely to be involved in regulating haptoglobin transcription during osteoclast differentiation. In vitro studies by Yanagihara et al. into the effects of human haptoglobin on bone resorption and prostanoid formation demonstrated that haptoglobin transcription negatively regulates osteoclast differentiation and modifies bone resorption processes [38]. Importantly, a proteomic analysis of the dry blood spots of cosmonauts after three months of SF also revealed significant changes in haptoglobin levels [25].
Our study identified altered concentrations of glutathione peroxidase 3 (GPx3 gene) at day 21 under both DI and HDBR conditions. This protein protects structural elements of bone tissue cells from oxidative damage by catalyzing the reduction of hydrogen peroxide, lipid peroxides, and organic hydroperoxides with glutathione, thereby shifting the balance between osteoblast and osteoclast activity. According to Föger-Samwald et al., increased expression of SOD2 and GPX3 suggests enhanced antioxidant activity in bone samples from individuals with osteoporosis and hip fractures [39]. It is hypothesized that osteoclast production of reactive oxygen species suppresses protective mechanisms of natural antioxidants. Concomitant oxidative stress may lead to bone loss and, consequently, to osteoporosis development [40].
Notably, when comparing proteomic results from ground-based experiments (using identical materials, blood plasma, and sampling timepoints) and prolonged SF (dried blood spot extracts, different sampling timepoints), common proteins were identified across different timepoints (7 days, 3 and 6 months) of a long-duration SF [18], and in blood plasma samples analyzed at day 21 of HDBR and DI. These include haptoglobin, apolipoprotein E, transthyretin, serotransferrin, and vitronectin. Their participation in bone tissue biological processes has been described above.
Table. Comparative assessment of proteomic regulation of bone tissue status during 21-day head-down bed rest (–6°) and 21-day dry immersion
|
Protein names |
Genes |
21-day HDBR |
21-day DI |
||
|
Protein levels in % relative to baseline |
Level of confidence in the observed changes (p-value) |
Protein levels in % relative to baseline |
Level of confidence in the observed changes (p-value) |
||
|
Apolipoprotein Е (APOE) |
APOE |
104.2 |
0.0024 |
– |
– |
|
Haptoglobin (Hp) |
HP |
105.3 |
0.0047 |
107.8 |
0.000018 |
|
Complement C5 alpha chain |
C5 |
115.1 |
0.0014 |
– |
– |
|
CD146 antigen (MUC18) |
MCAM |
95.7 |
0.0020 |
– |
– |
|
Extracellular glutathione peroxidase (GPx3) |
GPX3 |
106.5 |
0.0084 |
113.6 |
0.0000023 |
|
Angiotensinogen (AngII) |
AGT |
96.1 |
0.0035 |
– |
– |
|
Heparin cofactor II (HCII) |
SERPIND1 |
108.1 |
0.0038 |
– |
– |
|
Cholinesterase (CHLE) |
BCHE |
94.3 |
0.0039 |
– |
– |
|
Phosphatidylinositol-glycan-specific phospholipase D (PHLD) |
GPLD1 |
– |
– |
113.2 |
0.000015 |
|
Serum paraoxonase (PON1) |
PON1 |
– |
– |
115.2 |
0.00005 |
|
Fibronectin (FINC) |
FN1 |
– |
– |
108.7 |
0.00000013 |
|
Transthyretin (TTHY) |
TTR |
– |
– |
105.0 |
0.000013 |
|
Serotransferrin (TRFE) |
TF |
– |
– |
109.6 |
0.0000018 |
|
Vitronectin (VTNC) |
VTN |
– |
– |
109.9 |
0.0000065 |
Table prepared by the authors using their own data
Note: “–” the data are not presented due to the lack of statistically significant differences.
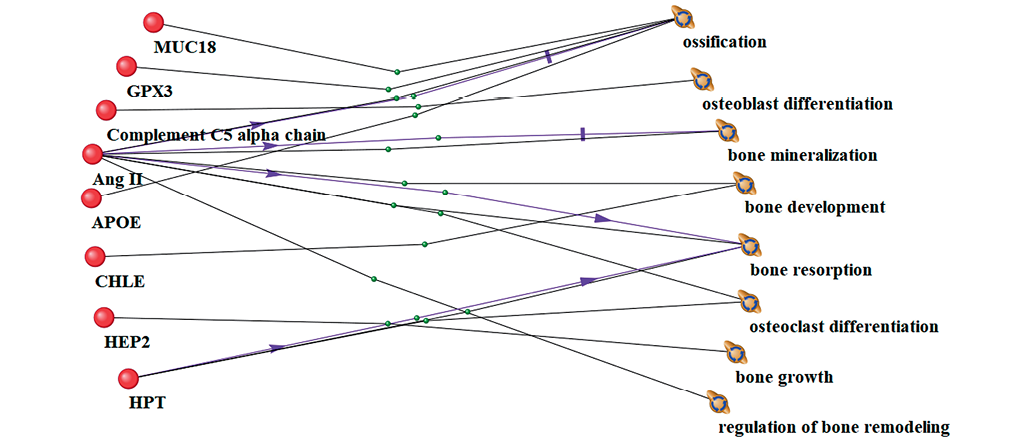
Figure prepared by the authors using their own data
Fig. 1. Interrelationship between significantly altered proteins and bone tissue processes under 21-day head-down bed rest (HDBR) conditions. Lines of different colors and labels indicate relationships between phenomena in peer-reviewed literature: black lines represent co-mention in scientific publications and association with biological processes; purple lines indicate stimulation or enhancement of biological process activity; green lines denote involvement or participation in biological processes, showing functional engagement in pathway mechanisms
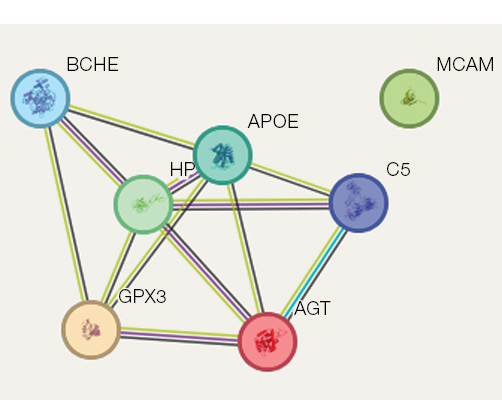
Figure prepared by the authors using their own data
Fig. 2. Protein–protein interactions associated with the regulation of bone tissue processes during 21-day head-down bed rest (HDBR). Protein–protein interaction lines are color-coded to indicate different types of evidence: light green represents co-mention in PubMed abstracts, indicating textual co-occurrence in scientific literature; crimson denotes experimentally determined interactions validated through laboratory methods; black indicates protein co-expression observed in transcriptomic or proteomic studies; light blue represents interactions curated from established biological databases
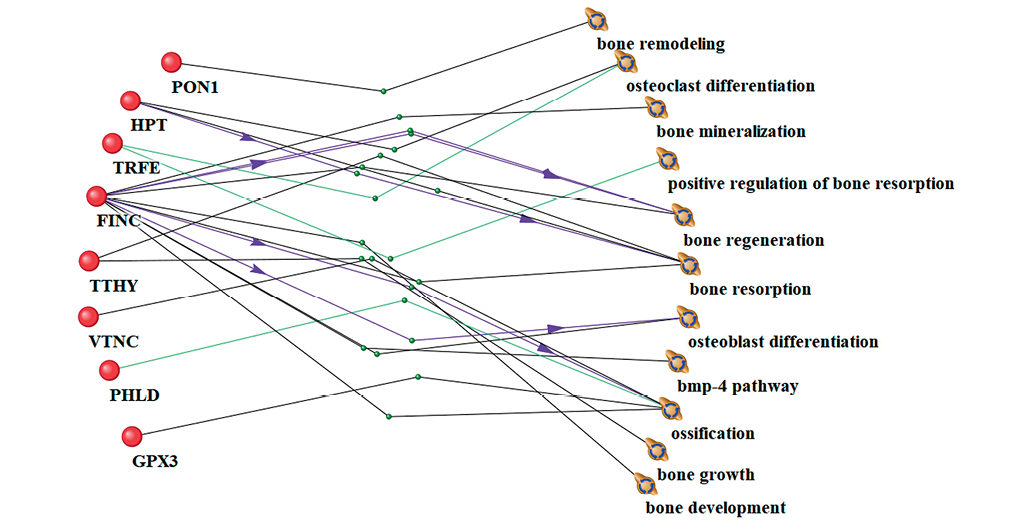
Figure prepared by the authors using their own data
Fig. 3. Interrelationship between significantly altered proteins and bone tissue processes under 21-day dry immersion (DI) conditions. Lines of different colors and labels indicate evidence-based relationships between biological phenomena: black lines represent co-occurrence in scientific literature and association with biological processes; purple lines indicate stimulation or enhancement of biological process activity; green lines denote functional involvement or participation in biological processes
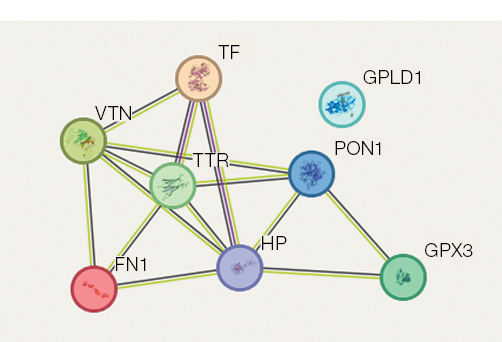
Figure prepared by the authors using their own data
Fig. 4. Protein–protein interactions associated with regulation of bone tissue processes during 21-day dry immersion (DI). Protein colors are assigned randomly by the visualization software. Interaction lines are color-coded as follows: light green indicates co-mention in PubMed abstracts (text mining evidence); crimson represents experimentally validated interactions; black denotes protein co-expression patterns
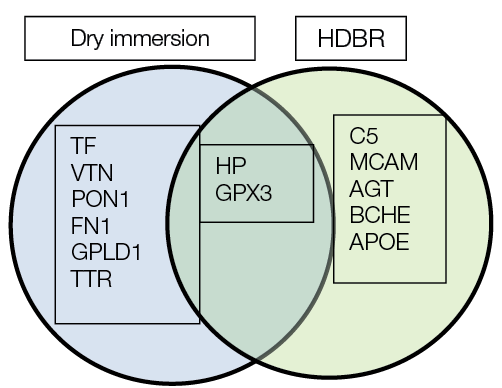
Figure prepared by the authors using their own data
Fig. 5. Common and differentially expressed proteins involved in bone tissue regulation under 21-day head-down bed rest (HDBR) and 21-day dry immersion (DI) conditions
CONCLUSION
In the present study, we conducted a comparative assessment of proteomic regulation in bone tissue during 21-day head-down bed rest (tilted at 6°) and 21-day dry immersion tests. Proteomic investigations of bone tissue regulation mechanisms in ground-based model studies identified nine proteins with significantly altered levels under HDBR conditions and eight proteins with statistically significant changes under DI conditions, all associated with the regulation of biological processes in bone tissue (osteogenesis, osteoblast differentiation, osteoclast differentiation, resorption, bone mineralization, bone development, and bone remodeling regulation).
Several proteins (in both HDBR and DI studies) formed protein–protein interaction networks, indicating potential co-expression. Different protein networks were associated with distinct biological effects of antiorthostatic hypokinesia and DI of the same duration. Two common proteins were identified — haptoglobin (Hp) and glutathione peroxidase 3 (GPx3) — participating in the regulation of bone tissue biological processes on day 21 of both DI and HDBR. Hp transcription negatively regulates osteoclast differentiation and alters bone resorption processes. GPx3 protects structural elements of bone tissue cells from oxidative damage, shifting the balance between osteoblast and osteoclast activity.
Bone system metabolism is a complex process involving numerous mechanisms. The proteomic level of regulation examined in our study extends the current understanding of the mechanisms underlying bone tissue changes at specific timepoints (day 21) of HDBR and DI. This duration is not sufficiently long for osteopenia to develop in healthy subjects; however, proteomic regulation of bone tissue biological processes significantly changes during this period. These findings indicate the involvement of both systemic and local protein regulators in bone tissue metabolic processes. For the first time, we revealed that the local bone renin-angiotensin-aldosterone system (RAAS) plays an important role in regulating bone tissue biological processes by day 21 of model studies. RAAS blockers may reduce the loss of bone mineral density (BMD) through AT1R, OPG/RANKL, and ACE2/Ang (1–7)/Mas cascades. Inhibition of acetylcholinesterase (AChEI) positively influences bone protection by suppressing osteoclast function through MAPK and Nfatc1 signaling pathways via downregulation of AChE. Changes in apolipoprotein E levels may reflect the activation of a protective biological process of osteogenesis in response to the duration of HDBR exposure.
Notably, significant alterations were observed in transport proteins involved in regulating biological processes of both bone resorption and growth. The production of reactive oxygen species (ROS) by osteoclasts and the role of oxidative stress in bone mass loss were also highlighted. Changes in the proteomic regulation of mineralization in the cancellous and cortical bone matrix are of great importance. The state of the matrix determines bone remodeling through structure formation, resorption, and osteogenesis.
Our findings draw attention to the primary proteomic mechanisms regulating biological processes in bone tissue in healthy individuals under exposure to 21-day HDBR and 21-day DI. These results hold significant implications for aerospace and clinical medicine.
1 ANDSystem Knowledge Base. https://www-bionet.sysbio.cytogen.ru/and/cell/#!/app/about
References
1. Navasiolava NM, Custaud MA, Tomilovskaya ES, Larina IM, Mano T, Gauquelin-Koch G, et al. Long-term dry immersion: review and prospects. European Journal of Applied Physiology. 2011;111(7):1235–60. https://doi.org/10.1007/s00421-010-1750-x
2. Hajj-Boutros G, Sonjak V, Faust A, Balram S, Lagace JC, St-Martin P, et al. Myths and Methodologies: Understanding the health impact of head down bedrest for the benefit of older adults and astronauts. Study protocol of the Canadian Bedrest Study. Experimental Physiology. 2024;109(5):812–87. https://doi.org/10.1113/EP091473
3. Hargens AR, Vico L. Long-duration bed rest as an analog to microgravity. Journal of Applied Physiology. 2016;120(8):891–903. https://doi.org/10.1152/japplphysiol.00935.2015
4. Oganov VS, Rakhmanov AS, Novikov VE, Zatsepin ST, Rodionova SS, Cann Ch. The state of human bone tissue during space flight. Acta Astronautica. 1991;23:129–33. https://doi.org/10.1016/0094-5765(91)90109-i
5. Baran R, Wehland M, Schulz H, Heer M, Infanger M, Grimm D. Microgravity-related changes in bone density and treatment options: a systematic review. Internation Journal of Molecular Sciences. 2022;23(15):8650. https://doi.org/10.3390/ijms23158650
6. Man J, Graham T, Squires-Donelly G, Laslett AL. The effects of microgravity on bone structure and function. NPJ Microgravity. 2022;8(1):9. https://doi.org/10.1038/s41526-022-00194-8
7. Larina IM, Morukov BV, Grigoriev AI. Circadian rhythms of human mineralotropic hormones during prolonged antiorthostatic hypokinesia. Human Physiology. 1999;25(6):89–95 (In Russ.).
8. Grigoriev AI, Morukov VV. 370-day antiorthostatic hypokinesia (tasks and general structure of the study). Space Biology and Aerospace Medicine. 1989;23(5):47–50 (In Russ.).
9. Austermann K, Baecker N, Zwart SR, Fimmers R, Stehle P, Smith SM, et al. Effects of antioxidant supplementation on bone mineral density, bone mineral content and bone structure in healthy men during 60 days of 6° head-down tilt bed rest: Results from a randomised controlled trial. Nutrition Bulletin. 2023;48(2):256–66. https://doi.org/10.1111/nbu.12619
10. Inoue M, Tanaka H, Moriwake T, Oka M, Sekiguchi C, Seino Y. Altered biochemical markers of bone turnover in humans during 120 days of bed rest. Bone. 2000;26(3):281–6. https://doi.org/10.1016/s8756-3282(99)00282-3
11. Tomilovskaya E, Shigueva T, Sayenko D, Rukavishnikov I, Kozlovskaya I. Dry immersion as a ground-based model of microgravity physiological effects. Frontiers in Physiology. 2019;10:284. https://doi.org/10.3389/fphys.2019.00284
12. Saveko A, Bekreneva M, Ponomarev I, Zelenskaya I, Riabova A, Shigueva T, et al. Impact of different groundbased microgravity models on human sensorimotor system. Frontiers in Physiology. 2023;14:1085545. https://doi.org/10.3389/fphys.2023.1085545
13. Kotov SA, Oganov VS, Skripnikova IA. Imitation of the early effects of weightlessness in human bone tissue in conditions of head down bed rest and dry immersion. Proceedings of the conference of young scientists. Moscow; 2003 (In Russ.).
14. Baecker N, Tomic A, Mika C, Gotzmann A, Platen P, Gerzer R, et al. Bone resorption is induced on the second day of bed rest: results of a controlled crossover trial. Journal of Applied Physiology. 2003;95(3):977–82. https://doi.org/10.1152/japplphysiol.00264.2003
15. Markin AA, Morukov BV, Zhuravleva OA, Zabolotskaya IV, Vostrikova LV, Lyapunova NA, et al. Dynamics of blood biochemical parameters in an experiment with 7-day immersion. Aerospace and Environmental Medicine. 2008;42:56– 9 (In Russ). EDN: RBLSWZ
16. Linossier MT, Amirova LE, Thomas M, Normand M, Bareille MP, Gauquelin-Koch G, et al. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PLoS One. 2017;12(8):e0182970. https://doi.org/10.1371/journal.pone.0182970
17. Brzhozovskiy AG, Kononikhin AS, Pastushkova LC, Kashirina DN, Indeykina MI, Popov IA, et al. The effects of spaceflight factors on the human plasma proteome, including both real space missions and ground-based experiments. International Journal of Molecular Sciences. 2019;20(13):3194. https://doi.org/10.3390/ijms20133194
18. Shukuri T, Nakai K, Tanaka S, Yamada S, Tokumoto M, Tsuruya K, et al. Angiotensin II receptor blockers and bone fracture in chronic kidney disease patients: the Fukuoka kidney disease Registry Study. Clinical and Experimental Nephrology. 2023;27(11):919–27. https://doi.org/10.1007/s10157-023-02385-3
19. Zhang Y, Wang L, Song Y, Zhao X, Wong MS, Zhang W. Renin inhibitor aliskiren exerts beneficial effect on trabecular bone by regulating skeletal renin-angiotensin system and kallikrein-kinin system in ovariectomized mice. Osteoporosis International. 2016;27:1083–92. https://doi.org/10.1007/s00198-015-3348-y
20. Zhang Y, Li XL, Sha NN, Shu B, Zhao YJ, Wang XL, et al. Differential response of bone and kidney to ACEI in db/db mice: A potential effect of captopril on accelerating bone loss. Bone. 2017;97:222–32. https://doi.org/10.1016/j.bone.2017.01.029
21. Mo C, Ke J, Zhao D, Zhang B. Role of the renin-angiotensinaldosterone system in bone metabolism. Journal of Bone and Mineral Metabolism. 2020;38(6):772–9. https://doi.org/10.1007/s00774-020-01132-y
22. Izbicka E, Dunstan CR, Horn D, Harris S, Adams R, Mundy GR. Effects of human tumor cell lines on local new bone formation in vivo. Calcified Tissue International. 1997;60(2):210–5. https://doi.org/10.1007/s002239900216
23. Li S, Teguh D, Wu D, Hu C, Inderjeeth CA, Xu J. Antidementia medication acetylcholinesterase inhibitors have therapeutic benefits on osteoporotic bone by attenuating osteoclastogenesis and bone resorption. Journal of Cellular Physiology. 2023;238(8):1823–35. https://doi.org/10.1002/jcp.31057
24. Souza LS, Rochette NF, Pedrosa DF, Lopes Magnago RF, Freire Filho TB, Vieira FLH, et al. Role of APOE gene in bone mineral density and incidence of bone fractures in brazilian postmenopausal women. Journal of Clinical Densitometry. 2018;21(2):227–35. https://doi.org/10.1016/j.jocd.2017.03.005
25. Pastushkova LH, Goncharova AG, Kashirina DN, Polyakov DN, Larina IM. Identification of proteomic markers included in molecular networks of disorders in the skeletal system during long-term space flights. Technologies of Living Systems. 2025;22(1):5–21 (In Russ.).
26. Bergdolt S, Kovtun A, Hägele Y, Liedert A, Schinke T, Amling M, et al. Osteoblast-specific overexpression of complement receptor C5aR1 impairs fracture healing. PLoS One. 2017;12(6):e0179512. https://doi.org/10.1371/journal.pone.0179512
27. Ignatius A, Schoengraf P, Kreja L, Liedert A, Recknagel S, Kandert S, et al. Complement C3a and C5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with IL-1β. Journal of Cellular Biochemistry. 2011;112(9):2594–605. https://doi.org/10.1002/jcb.23186
28. Pimenta-Lopes C, Sánchez-de-Diego C, Deber A, EgeaCortes A, Valer JA, Alcala A, et al. Inhibition of C5AR1 impairs osteoclast mobilization and prevents bone loss. Molecular Therapy. 2023;31(8):2507–23. https://doi.org/10.1016/j.ymthe.2023.04.022
29. Kunimatsu R, Rikitake K, Yoshimi Y, Putrani NAR, Hayashi Y, Tanimoto K. Bone differentiation ability of CD146-positive stem cells from human exfoliated deciduous teeth. International Journal of Molecular Sciences. 2023;24(4):4048. https://doi.org/10.3390/ijms24044048
30. Xiong W, Shu XL, Huang L, He SQ, Liu LH, Li S, et al. Bioinformatics analysis and experimental validation of differential genes and pathways in bone nonunions. Biochemical Genetics. 2024;62(6):4494–517. https://doi.org/10.1007/s10528-023-10633-0
31. Zhang H, Chen X, Xue P, Ma X, Li J, Zhang J. FN1 promotes chondrocyte differentiation and collagen production via TGF-β/PI3K/Akt pathway in mice with femoral fracture. Gene. 2021;769:145253. https://doi.org/10.1016/j.gene.2020.145253
32. Dakkumadugula A, Pankaj L, Alqahtani AS, Ullah R, Ercisli S, Murugan R. Space nutrition and the biochemical changes caused in Astronauts Health due to space flight: A review. Food Chemistry: X. 2023;20:100875. https://doi.org/10.1016/j.fochx.2023.100875
33. O’Leary TJ, Jackson S, Izard RM, Walsh NP, Carswell AT, Oliver SJ, et al. Iron status is associated with tibial structure and vitamin D metabolites in healthy young men. Bone. 2024;186:117145. https://doi.org/10.1016/j.bone.2024.117145
34. Nakashima M, Suzuki A, Hashimoto K, Yamashita M, Fujiwara Y, Miyamoto Y. Vitronectin regulates osteoclastogenesis and bone remodeling in a mouse model of osteoporosis. Anatomy and Cell Biology. 2024;57(2):305–15. https://doi.org/10.5115/acb.23.251
35. Aydın M, Avcı E. The role of oxidative stress and antioxidants in older individuals with osteoporotic hip fractures. Turkish Journal of Trauma and Emergency Surgery. 2025;31(1):9–14. https://doi.org/10.14744/tjtes.2024.89335
36. Cao J, Zhou A, Zhou Z, Liu H, Jia S. The role of GPLD1 in chronic diseases. Journal of Cellular Physiology. 2023;238(7):1407–15. https://doi.org/10.1002/jcp.31041
37. Li XS, Zhang JR, Zhao YL, Li Y, Sun Y, Liu T, et al. Reduced prealbumin is associated with bone mineral density in women with osteoporosis. Nutrition. 2017;33:338–42. https://doi.org/10.1016/j.nut.2016.08.002
38. Yanagihara Y, Inoue K, Saeki N, Sawada Y, Yoshida S, Lee J, et al. Zscan10 suppresses osteoclast differentiation by regulating expression of Haptoglobin. Bone. 2019;22:93–100. https://doi.org/10.1016/j.bone.2019.02.011
39. Föger-Samwald U, Vekszler G, Hörz-Schuch E, Salem S, Wipperich M, Ritschl P, et al. Molecular mechanisms of osteoporotic hip fractures in elderly women. Experimental Gerontology. 2016;73:49–58. https://doi.org/10.1016/j.exger.2015.11.012
40. Zhang W, Dong R, Diao S, Du J, Fan Z, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Research and Therapy. 2017;8(1):30. https://doi.org/10.1186/s13287-017-0485-6
About the Authors
L. Kh. PastushkovaRussian Federation
Ludmila Kh. Pastushkova - Dr. Sci. (Biol.)
Moscow
A. G. Goncharova
Russian Federation
Anna G. Goncharova - Dr. Sci. (Med.)
Moscow
D. N. Kashirina
Russian Federation
Daria N. Kashirina - Cand. Sci. (Biol.)
Moscow
I. M. Larina
Russian Federation
Irina M. Larina - Dr. Sci. (Med.)
Moscow
Supplementary files
Review
For citations:
Pastushkova L.Kh., Goncharova A.G., Kashirina D.N., Larina I.M. Comparative assessment of proteomic regulation of bone tissue during 21-day head-down bed rest (–6°) and 21-day dry immersion. Extreme Medicine. 2025;27(4):558-568. https://doi.org/10.47183/mes.2025-296

















