Scroll to:
Clonal haematopoiesis and ionizing radiation: risks for hematological malignancies and somatic diseases
https://doi.org/10.47183/mes.2024-26-4-5-12
Abstract
Introduction. The influence of radiation-induced genetic instability on the formation of clonal expansion is a relevant problem in health monitoring and preventive diagnostics of oncohematological and somatic pathology in individuals exposed to long-term low-dose anthropogenic irradiation, such as nuclear industry workers and radiation diagnostics doctors.
Objective. Identification of possible application points of preventive diagnostics of genome instability markers and clonal hematopoiesis in groups of individuals exposed to long-term low-dose anthropogenic irradiation.
Results and discussion. Genetic instability in genes of epigenetic regulation (DNMT3A, TET2, ASXL1), signaling pathways and cell proliferation (JAK2, FLT3), DNA repair regulators (TP53, PPM1D), RNA splicing factors (SF3B1, SRSF2) most often initiates clonal hematopoiesis, which is realized more frequently by myeloid and less frequently by lymphoid neoplasia. The influence of clonal hematopoiesis on the development of somatic diseases is mediated by the combined effect of carrying these mutations and the processes of chronic inflammation. Low-dose ionizing radiation is capable of initiating clonal expansion mainly due to mutations in DNMT3A and TET2 genes. There is a lack of studies on the assessment of increased morbidity against the background of clonal hematopoiesis in groups of occupational risk of low-dose ionizing radiation exposure (workers in the nuclear industry and doctors of radiation diagnostics), which requires further study.
Conclusions. Studies aimed at identifying risk markers of morbidity growth in the setting of clonal hematopoiesis in groups of workers exposed to long-term anthropogenic action of low-dose ionizing radiation form the basis for developing cohort-oriented programs of disease prevention in these individuals.
Keywords
For citations:
Zherniakova A.A., Krysiuk O.B., Kunevich Ye.O. Clonal haematopoiesis and ionizing radiation: risks for hematological malignancies and somatic diseases. Extreme Medicine. 2024;26(4):5-12. https://doi.org/10.47183/mes.2024-26-4-5-12
INTRODUCTION
Research into the radiation-induced genetic instability and its interrelation with clonal expansion is a relevant direction with regard to health monitoring of nuclear industry workers and radiologists. Identification of risk factors in the emergence of adverse consequences in these categories of individuals, along with dispensary monitoring and timely prophylaxis, may contribute to combating the development of related pathologies [1].
On a daily basis, human beings are exposed to low-dose ionizing radiation (IR) emitted both by natural (environmental radionuclides found in the atmosphere, soil, and water) and artificial or man-made sources. The cumulative effect of all types of natural radiation is defined as the natural radioactive background. The global average values of such radiation in different regions of the world range from 2.4 to 4.0 mSv/year, with the average level in the Russian Federation comprising 3.36 (from 2.10 to 8.60) mSv/year [1–3].
The impact of IR on humans is realized through the influence of the natural radioactive background, occupational exposure at the workplace, as well as when receiving medical care (medical exposure). The widespread use of IR in medical and other industries, primarily in the nuclear power industry, contributes to an increase in radiation exposure of workers. Thus, for a certain list of industries, where additional anthropogenic exposure to IR is possible, the limit of permissible effective IR dose reaches 5 mSv/year for Group B personnel and 20 mSv/year for Group A personnel [3].
At present, the immediate and delayed consequences of human exposure to high-dose IR in technogenic catastrophes, characterized by critical changes leading to defects in the functioning of organs and systems, have been studied and described in sufficient detail. At the same time, exposure to low-dose IR, although without clinical manifestations of radiation damage, may also induce damage at the genetic and epigenetic levels, with the effect depending on the radiation dose in a non-monotonic polymodal character. The recent data points to the effect of low-dose radiation on genome instability, modifying cellular and tissue processes. This may eventually contribute to changes in the body sensitivity to the action of additional non-radiation factors [4–6].
Over the past decade, research interest has been revolving around clonal hematopoiesis (CH), considering it as a biological condition predisposing to the development of malignant blood disorders (MBD), solitary tumors, cardiovascular pathology, autoimmune diseases, and other pathologies [7–9].
This work aims to identify the possibility of preventive diagnostics of genome instability markers and CH markers in work groups exposed to long-term low-dose technogenic irradiation.
RESULTS AND DISCUSSION
General understanding about clonal hematopoiesis
Hematopoietic cells of the bone marrow (BM) exhibit the highest proliferative potential. The processes of blood cell proliferation and differentiation in BM are carried out continuously. About one trillion blood cells are formed daily. The basis of hematopoiesis are hematopoietic stem cells (HSCs), which have the ability to differentiate into all mature cells of peripheral blood. It was first postulated by Prof. A.A. Maximov in 1909. The hematopoiesis model proposed by Maximov was confirmed by experimental data obtained in the era of active study of radiation effects on the human body [10].
Over the past decades, our understanding of the hematopoiesis system has been significantly extended [10–13]. Thus, numerous studies have advanced the idea of continuous differentiation landscapes having a few or no discrete differentiation stages and smooth cell state transitions. In this context, cells in a heterogeneous pool of progenitors differentiate along multiple potential trajectories that contain some branching points determining the fate of a particular cell. However, the notion that the progeny of a single HSC represents a clone of a particular HSC remains unchanged. The normal hematopoietic system is polyclonal that assumes production of “own” cell clones by different HSCs and maintenance of their number as well as the ratio of cells with varied degrees and directions of differentiation in BM within stable limits. Hematopoietic cells with a high proliferative activity are characterized by accumulation of genetic “breaks” with increasing number of divisions [14].
Upon human aging, physiological quantitative and qualitative changes occur in hematopoiesis, including a decrease in the total proliferative HSC activity with a relative increase in their total number, an increase in the erythroid precursors number, a decrease in the lymphoid precursors number with age-related changes in the immune system function. In general, hematopoiesis tends to oligoclonality [15]. Genetic and epigenetic processes most likely underlie the age-related changes in BM. The age-related decrease of HSC regenerative potential [16], specific changes in expression of transcription regulator genes, accumulation of somatic mutations [17], and pronounced shortening of telomeric chromosome parts [18] were noted. In addition, in elderly people, increased expression of genes involved in the regulation of apoptosis and inflammation in the hematopoiesis system was detected; however, in young people, the activity of genes regulating the processes of proliferation and metabolic activity of hematopoietic progenitor cells is high. All the described trends are manifestations of regular processes characterizing changes in hematopoiesis in the process of aging of the organism [19].
At the same time, the accumulation of mutations can lead to a CH with activated proliferation of one HSC or more differentiated hematopoietic progenitor cell and with formation of a progeny clone carrying gene mutations. While CH is not an independent nosology, it can be an intermediate stage between normal hematopoiesis and MBD [20]. It has been shown that CH can arise as a result of neutral drift or directed selection. In the case of neutral drift, all cell clones initially have equal chances of their contribution to the formation of the pool of self-renewing HSCs; the decisive influence is exerted by random processes, such as depletion of the stem cell pool. In the case of directed selection, somatic changes influence the selective growth advantage in certain HSC clones relative to others, with the subsequent clonal expansion [21].
In 2014, two independent groups published the results of large-size studies conducted using next-generation sequencing (NGS). According to the data obtained, CH is associated with somatic mutations most frequently occurring in three genes of epigenetic regulation of transcription. These include DNMT3A — a gene encoding a protein that performs de novo methylation; TET2 — encoding a protein that catalyzes the conversion of the modified DNA base methylcytosine into 5-hydroxymethylcytosine and participates in the transcription process regulation; and ASXL1 — encoding a nuclear protein involved in the epigenetic regulation of gene expression and in the process of chromatin remodeling. The obtained results were confirmed in a study of groups of patients with different types of MBD and solid neoplasms [22][23]. The work by M. Xie et al. convincingly demonstrated that mutations in DNMT3A, TET2, ASXL1, and TP53 genes occurred most frequently and almost in all analyzed nosological subgroups [24].
In 2015, on the basis of the accumulated data, the term “clonal hematopoiesis of indeterminate potential” (CHIP) was coined. Thus, CHIP is defined in the presence of a hematopoietic cell clone with a gene mutation associated with the risk of MBD in the absence of cytopenia and criteria of other hematological diseases, according to the classification of the World Health Organization. The allele load level of the indicated gene should be not less than 2% (the value is accepted as the minimum clinically significant threshold level for the next-generation sequencing method — NGS), and for X-linked genes in men — not less than 4% [25][26]. The frequency of mutation detection increases with age, and their presence is associated with an increased risk of MBD. It was determined that about 15–20% of people over 70 years of age without oncohematological diseases have somatic mutations associated with an increased risk of oncopathology [27]. Later, the term “age-related clonal haematopoiesis” was proposed for such changes, which is defined in elderly patients with a somatic mutation in genes regardless of its clinical significance and allele load level, as well as with no changes in the hemogram and MBD criteria [23][27].
The term “clonal haematopoiesis of oncogenic potential” (CHOP) was introduced to designate the state of carrying mutations that can act as a direct driver of MBD [28][29]. The allocation of such a category and the approach to the division of genes according to their direct role in the development of MBD (i.e., their oncogenic potential) is currently rather tentative and debatable, thus requiring data accumulation and analysis.
The term “clonal cytopenia of undetermined significance” can also be found in the scientific literature, which is characterized by the presence of somatic mutations in the gene(s) associated with MBD, with an allele burden of at least 2% (or 4% in men in the case of X-linked genes mutations); absence of criteria for MBD, other causes of cytopenia and molecular aberrations; persistent cytopenia in more than one hematopoietic growth (hemoglobin less than 100 g/L, neutrophils less than 1.8×10⁹/L, platelets less than 100×10⁹/L) for at least four months. The term “idiopathic cytopenia of undetermined significance” (idiopathic cytopenia of undetermined significance) is also proposed to be used in the presence of persistent cytopenia in more than one hematopoietic growth and in the absence of criteria for myeloid neoplasm and other blood system diseases [30].
Clonal hematopoiesis and oncohematological diseases
It has been proven that individuals with CH are more likely to show signs of genetic instability due to somatic mutations in epigenetic regulation genes (DNMT3A, TET2, ASXL1), RNA splicing genes (SF3B1, SRSF2, ZRSR2), signaling pathways and cell proliferation (JAK2, FLT3), genes related to metabolism and cell differentiation (IDH1, IDH2), and in DNA repair regulation genes (TP53, PPM1D). In comparison with the overall population, such individuals are also frequently diagnosed with cytopenia of unclear etiology. Mutations in DNMT3A, TET2, and ASXL1 genes account for about 80% of CHIP cases [22][23].
Mutations characteristic of myelodysplastic syndrome (MDS), myeloproliferative neoplasms, and acute myeloid leukemia are observed in JAK2, PPM1D, TP53, SRSF2, and SF3B1 genes, being less frequent [26]. The frequency of detection of these mutations is much higher than the incidence of MBD; nevertheless, CH can be considered as an event preceding the development of hemoblastosis. Signs of CH are revealed in 50% of patients with aplastic anemia; CH-specific DNMT3A and ASXL1 gene mutations are found in 15% of patients [31][32]. Another example of CH with its transformation into a pathological process is paroxysmal nocturnal hemoglobinuria. In this disease, the clone arisen from HSCs with glycosyl-phosphatidylinositol deficiency is less susceptible to T-cell-mediated destruction in comparison with normal HSCs [30][33].
In addition, CH with point mutations or structural variations, such as gene copy number changes, leads to a ten-fold increase in the risk of MBD. The role of antitumor immunity disorders in the transformation of CH into MBD requires further investigation [22]. At the same time, the presence of CHIP is associated with a 13-fold increase in the risk of hemoblastosis, with the frequency of its occurrence in 0.5–1% of patients per year [8].
In most cases, CHIP is benign, especially when the clone size is small and multiple, without driver mutations [20]. The main risk factors for the development of the disease (not only hematologic but also somatic) into CHIP include: a significant clone size (10% or more) and its growth acceleration, clonal changes in more than one cell line, multiple driver mutations, TP53 gene mutations, and chromosomal aberrations [34].
Until recently, the accumulated information and expansion of detectable genetic mutations spectrum has not allowed differentiation of mutations that initiate the onset of CH from those that are an early event in the development of MBD, as well as to identification of second-order mutations that make the most significant contribution during the disease progression. Thus, the isolation of driver mutations, passenger mutations, and background and cooperating mutations has been proposed [20][22]. The works published in 2024 indicate that methods have already been developed to identify driver mutations, such as “a method for enriching nonsynonymous mutations over neutral synonymous mutations” or machine learning based algorithms [35][36].
Thus, in accordance with modern concepts, mutations of genes associated with CHIP is a predisposing factor in MBD. Appearance of such mutations leads to transformation of normal HSC into “pre-leukemia” neoplastic HSC. Altered HSC gives rise to small subclones and, itself, does not practically differ from normal HSC. The frequency of mutation detection increases with age, being a natural biological process accompanying aging. However, “pre-leukemia” HSC has an increased risk for transformation into leukemia HSC at acquisition of additional molecular-genetic damages. Factors in such transformations require further study. Classification of genetic breakages according to their oncogenic potential is also an area of interest for current research. The CH model presented in Fig. 1 summarizes our ideas about driver, cooperating, passenger, and background mutations, as well as the causes of genetic instability that determine clonal evolution with the development of MBD.
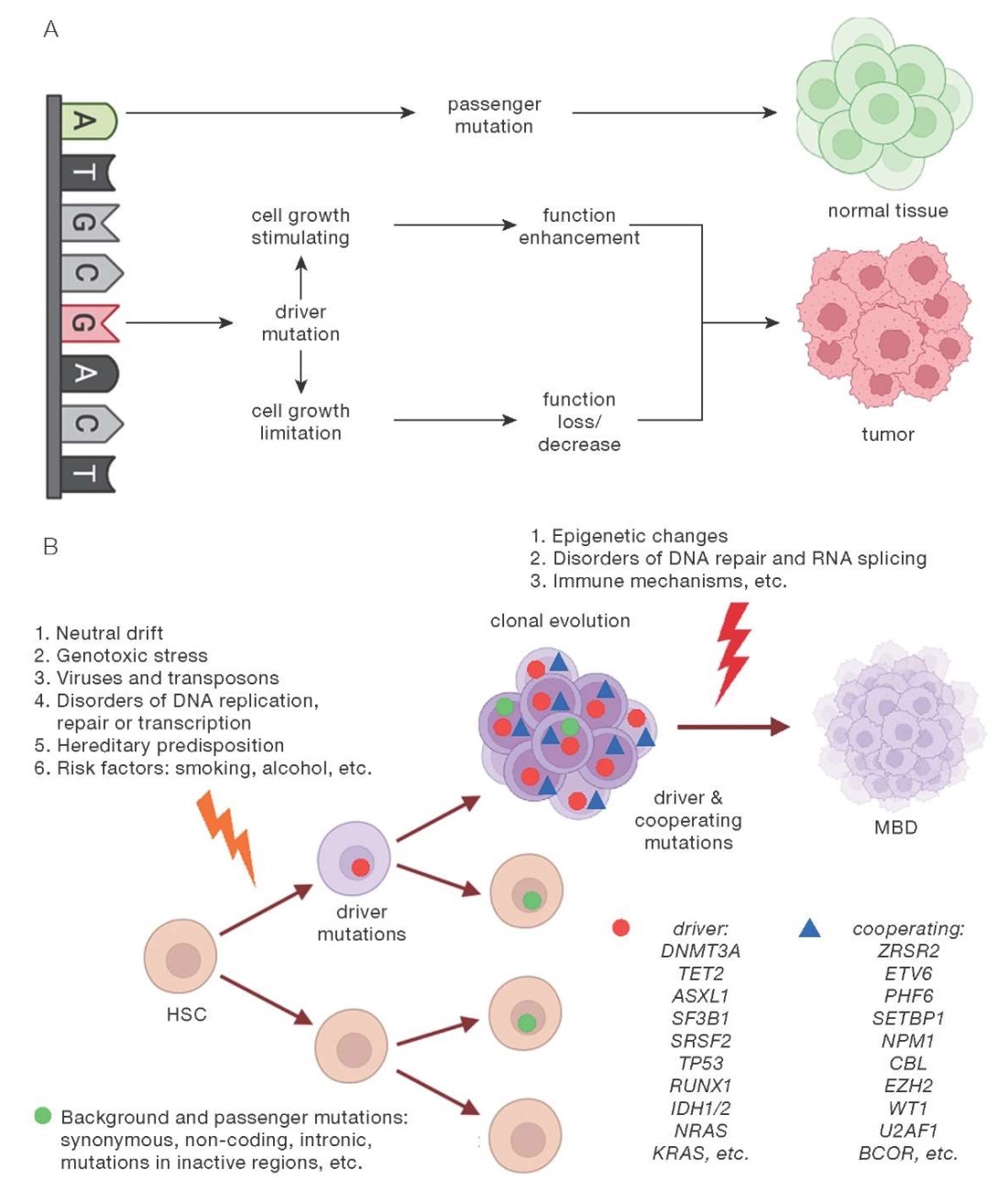
Figure prepared by the authors using data from [24]
Fig. 1. Model of clonal hematopoiesis: realization of passenger (A) and driver (B) mutations activity
Clonal hematopoiesis and somatic pathology
The development of CHIP is an independent risk factor concerning not only various hemoblastoses, but also cardiovascular diseases, Alzheimer’s disease, type 2 diabetes mellitus, thrombosis, autoimmune processes, and other pathologies [7]. The clonal expansion of HSC encounters no anatomical restrictions, with clone cells circulating in the bloodstream in large numbers [21]. Thus, CH is associated with an increased risk of developing both MBD (more often myeloid and less often lymphoid) and various somatic pathologies [21][37].
A number of publications have convincingly demonstrated that CHIP correlates with the risk of acute leukemia, MDS, and other hemoblastoses, as well as with such adverse cardiovascular events, as acute cerebral circulatory disorder and acute myocardial infarction [38–40]. Two independent meta-analysis studies also showed the link of CHIP with a high risk of atherosclerosis and cardiovascular disease [40][41].
The presence of CHIP contributes to the acceleration of atherosclerotic vascular lesions. This fact was confirmed in animal models, and similar conclusions were obtained for humans [41–43]. The mutations characteristic of CHIP accelerate the atherosclerotic process, which was noted even under a slight increase in their allele load [41]. An experimental model of CHIP in mice showed that an atherosclerotic lesion of the aorta was 60% more pronounced in the experimental animals compared to the control group [43]. Such modeling of CHIP showed its exceptional reproducibility in other animal experiments [43][44]. Similar results were obtained when modeling CHIP associated with JAK2V617F mutation. The presence of this mutation in animals caused an increase in erythrophagocytosis in atherosclerotic plaques, which accelerated their destabilization [45]. In addition, this mutation was associated with a higher frequency of thrombosis in atherosclerotic plaques [45][46].
Clonal hematopoiesis of indeterminate potential increases not only the risk of acute cardiovascular events, but also aggravates the course of heart failure (HF) [40][45]. In a study by Sano S et al., mice were transplanted with 10% Tet2-/- BM cells, and the association of inactivated driver gene with high incidence of HF development was found [43]. A detailed transcriptomic analysis of heart tissues in animals demonstrated an increased expression of NLRP3-inflammasome-related genes, as well as IL1B gene. The use of NLRP3 inhibitor decreased the incidence of cardiac remodeling and the risk of developing HF in mice [44][47][48]. Thus, driver mutations of CHIP were demonstrated to intensify the proinflammatory response leading to the development of cardiovascular pathology [7][45][48].
At the same time, new data continues to emerge, stimulating further studies regarding the role of CHIP in the development of various variants of the course of type 2 diabetes [7], Alzheimer’s disease [7][49], autoimmune diseases [7][50], thrombosis [7][46], and other pathologies. The decisive role in somatic pathology is attributed to the combination of effects of somatic mutations characteristic of CHIP with chronic inflammatory process, prolonged hyperactivation of the immune system, concomitant pathology and its therapy, lifestyle, as well as the impact of external factors [46].
Ionizing radiation as an inductor of genetic instability and clonal hematopoiesis
The action of IR leads to disruption of repair processes, interphase or mitotic reproductive cell death, and increasing deficit of differentiated cells with the formation of deterministic effects of irradiation. The latter consist in the direct damaging effect of IR on cells and tissues, manifesting themselves only after irradiation above the threshold dose with clinically significant consequences, both deterministic (tissue reactions) and stochastic. The degree of damage severity increases rapidly as the radiation dose accumulates. [51][52].
When exposed to low-dose IR, stochastic effects develop. Low doses in this case are commonly understood as follows: a single equivalent dose of up to 0.1 Sv or 10 rem; an absorbed dose of up to 0.1 Gy or 10 rad; an effective equivalent dose of up to 0.1 Sv/year, which approximately corresponds to the exposure dose of 750 µR/h [1][6]. In case of such exposure, when an insignificant damage to cells under the IR influence occurred but was completely eliminated by regenerative processes, the cells retain viability but acquire genetic mutations. The probability of such damage at a single exposure to low doses of II is minimal, although sharply increasing with increasing the effective equivalent dose and duration of exposure. Consequently, stochastic effects are characterized by the absence of a dose threshold and are assumed to occur with a probability linearly proportional to the influencing IR dose. Stochastic effects include the development of MBD and solid tumors, as well as hereditary pathologies [4][6][51].
Thus, the main reaction of the body to the chronic effect of low-dose IR is a disorder of genome stability and regulatory processes. In the setting of genetic instability, various reactions of the organism to the impact of external factors, up to death, are possible. An increase in the number of chromosomal aberrations may precede the development of secondary immunodeficiency, premature aging, neoplasia, including MBD, as well as somatic pathologies. Low-dose IR is a stress factor for the organism, with the distant consequences of its long-term chronic exposure consisting in the depletion of compensatory capabilities of the organism [7][52].
The number of publications on occupational exposure effects, including low-dose IR in a certain professional contingent (workers in the nuclear industry, radiologists, etc.) is currently growing. In this regard, a study by Jasra S et al published in 2022 seems noteworthy. The researchers analyzed the effects of chemical substances and dust particulate matter on 481 rescue workers involved in combating the disaster in the World Trade Center on September 11, 2001. A significant increase in the CH risk in these individuals was determined, with the most frequent mutations affecting the DNMT3A and TET2 genes. The frequency of their detection increased with age in comparison with 255 employees from the comparison group [53].
Another large-scale study, conducted in 2019 among atomic bombing survivors without an MBD diagnosis, found the radiation influence to result in blood cell clonal expansion. This led to a long-term increase in circulating monocytes in the group of people older than 60 years [54]. Also in 2022, the results of a study analyzing the presence of CH driver genes mutations in NASA astronauts were published. The study identified 34 nonsynonymous mutations in 17 driver genes, with the highest frequency of occurrence in the TP53 and DNMT3A genes [55].
A recent research focus has been the occupational IR impact on oncologic and cardiovascular pathologies in employees of the nuclear industry and radiologists. Thus, a large meta-analysis found the absorbed IR dose, above which the mortality from circulatory system diseases in these categories of workers increases, to be equal to 0.5 Gy [56].
A meta-analysis study (data from 15 countries) on the total mortality and mortality from all malignant neoplasms for workers in the nuclear industry, as well as for workers in contact with the most toxic heavy metals and chemical compounds, revealed no obvious increase in mortality from all malignant neoplasms compared to the population [57]. In a meta-analysis of data on the risks of cardiovascular pathologies, the authors also noted no significant differences with the population [58]. However, in the context of the effect of low-dose IR on the CH in these categories of individuals, no information is currently presented in large-scale works on the identification of mutations associated with CH. The majority of domestic studies are centered around the characteristics of IR and a retrospective analysis of morbidity, as well as the survival of employees exposed to long-term low-dose IR. Consequently, the possibility of identifying mutations associated with CH is of greatest interest for current research.
CONCLUSION
To date, the association of CH with natural aging processes has been well established. The influence of CHIP on the increased risk of hemoblastosis, somatic pathology, and overall mortality has been documented. Genetic instability in the DNMT3A, TET2, ASXL1. JAK2, TP53, PPM1D, SF3B1, and SRSF2 genes, which most often initiate CH in combination with chronic inflammation and increased immune activation, is associated with these diseases.
At the same time, there is a lack of comprehensive information on the risks of transition of clinically silent CHIP into disease. Timely screening aimed at identification of factors correlating with unfavorable outcomes can facilitate timely identification of people with an increased risk of pathology development.
Research into the influence of radiation-induced genetic instability on the formation of clonal expansion is relevant for health monitoring and preventive diagnosis of oncohematological and somatic pathologies in individuals with long-term low-dose anthropogenic exposure (nuclear industry workers and radiologists).
Further studies should elucidate the role of CH and CHOP in various pathologies, determining the possibilities of therapeutic effects aimed at preventing unfavorable course of the disease and increasing life expectancy.
Authors’ contributions. All the authors confirm that they meet the ICMJE criteria for authorship. The most significant contributions were as follows. Anastasiia A. Zherniakova — concept of the article, literature review, collection and analysis of literary sources, writing the text; Yevgeny O. Kunevich — literature review, collection and analysis of literary sources, writing the text and the figure; Oleg B. Krysiuk — concept of the article, literature review, collection and analysis of literary sources, editing the article and final approval of the manuscript.
References
1. Marennyy AM, Kiselev SM, Semenov SYu. On the problem of protection of the russian population from natural sources of ionizing radiation. part 2. the development of approaches and practical activities. Extreme Medicine. 2019;21(3):371–82 (In Russ). EDN: CTYGIX
2. Puchkov VА, ed. Civil protection. Мoscow: FSBI VNII GOChS; 2015 (In Russ.).
3. Barkovsky AN, Akhmatdinov RR, Akhmatdinov RR, Baryshkov NK, Biblin AM, Bratilova AA, et al. Radiation doses to the population of the russian federation in 2020. Radiation Hygiene. 2021;14(4):103–13 (In Russ.). https://doi.org/10.21514/1998-426X-2021-14-4-103-113
4. Kostryukova NK, Karpin VA. Biological effects of low doses of ionizing radiation. BMJ. 2005;50(1):17–22 (In Russ.).
5. Kuznetsova EA, Zaichkina SI, Sirota NP, Abdullaev SA, Rozanova OM, Aptikaeva GF, et al. Induction of DNA damage in blood leukocytes and cytogenetic damage in polychromatophilic erythrocytes of the bone marrow of mice and their descendants by rare and dense ionizing radiation. Radiation Biology. Radioecology. 2014;54(4):341–9 (In Russ). https://doi.org/10.7868/S0869803114040080
6. Zhizhina GP. The effects of low doses of low-intensity ionizing radiation on DNA structure and function. Radiation Biology. Radioecology. 2011;51(2):218–28. EDN: NSYSVF
7. Cacic AM, Schulz FI, Germing U, Dietrich S, Gattermann N. Molecular and clinical aspects relevant for counseling individuals with clonal hematopoiesis of indeterminate potential. Front. Oncol. 2023;13:1303785. https://doi.org/10.3389/fonc.2023.1303785
8. Jaiswal S. Clonal hematopoiesis and nonhematologic disorders. Blood. 2020;136(14):1606–14. https://doi.org/10.1182/blood.20190009899
9. Reed SC, Croessmann S, Park BH. CHIP Happens: Clonal Hematopoiesis of Indeterminate Potential and Its Relationship to Solid Tumors. Clin Cancer Res. 2023;29(8):1403–11. https://doi.org/10.1158/1078-0432.CCR-22-2598
10. Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012:10(2):120–36. https://doi.org/10.1016/j.stem.2012.01.006
11. Watcham S, Kucinski I, Gottgens B. New insights into hematopoietic differentiation landscapes from single-cell RNA sequencing. Blood. 2019;133(13):1415–26. https://doi.org/10.1182/blood-2018-08-835355
12. Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015; 125(17): 2605–13. https://doi.org/10.1182/blood-2014-12-570200
13. Nimmo RA, May GE, Enver T. Primed and ready: understanding lineage commitment through single cell analysis. Trends. Cell. Biol. 2015;25(8):459–67. https://doi.org/10.1016/j.tcb.2015.04.004
14. Lee-Six H, Øbro NF, Shepherd MS, Grossmann S, Dawson K, Belmonte M, et al. Population dynamics of normal human blood inferred from somatic mutations. Nature. 2018;561(7724):473–8. https://doi.org/10.1038/s41586-018-0497-0
15. Mitchell E, Spencer Chapman M, Williams N, Dawson KJ, Mende N, Calderbank EF, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606(7913):343–50. https://doi.org/10.1038/s41586-022-04786-y
16. Vijg J. From DNA damage to mutations: All roads lead to aging. Ageing Res Rev. 2021;68(101316). https://doi.org/10.1016/j.arr.2021.101316
17. Vijg J. Pathogenic Mechanisms of Somatic Mutation and Genome Mosaicism in Aging. Cell. 2020;182(1):12–23. https://doi.org/10.1016/j.cell.2020.06.024
18. Holstege H, Pfeiffer W, Sie D, Hulsman M, Nicholas TJ, Lee CC, et al. Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 2014;24(5):733–42. https://doi.org/10.1101/gr.162131.113
19. Ainciburu M, Ezponda T, Berastegui N, Alfonso-Pierola A, VilasZornoza A, San Martin-Uriz P., et al. Uncovering perturbations in human hematopoiesis associated with healthy aging and myeloid malignancies at single-cell resolution. Elife. 2023;12:e79363. https://doi.org/10.7554/eLife.79363
20. Kashlakova AI, Biderman BV, Parovichnikova E.N. Clonal hematopoiesis and acute myeloid leukemia. Oncohematology. 2023;18(3):92–101 (In Russ.). https://doi.org/10.17650/1818-8346-2023-18-3-92-101
21. Petinati NA, Drize NJ. Clonal hematopoiesis and its role in the development of hematological diseases. Russian Journal of Hematology and Transfusiology. 2021;66(4):580–92n (In Russ.). https://doi.org/10.35754/0234-5730-2021-66-4-580-592
22. Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371(26):2477–87. https://doi.org/10.1056/NEJMoa1409405
23. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014; 371(26):2488–98. https://doi.org/10.1056/NEJMoa1408617
24. Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014;20(12):1472–8. https://doi.org/10.1038/nm.3733
25. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am. Soc. Hematol. Educ. Program. 2018;2018(1):264–9. https://doi.org/10.1182/asheducation-2018.1.264
26. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. https://doi.org/10.1182/blood-2015-03-631747
27. Cooper JN, Young NS. Clonality in context: hematopoietic clones in their marrow environment. Blood. 2017:130(22):2363–72. https://doi.org/10.1182/blood-2017-07-794362
28. Valent P, Kern W, Hoermann G, Milosevic Feenstra JD, Sotlar K, Pfeilstöcker M, et al. Clonal hematopoiesis with oncogenic potential (CHOP): separation from CHIP and roads to AML. Int. J. Mol. Sci. 2019;20(3):789. https://doi.org/10.3390/ijms20030789
29. Cappelli LV, Meggendorfer M, Baer C, Nadarajah N, Hutter S, Jeromin S, et al. Indeterminate and oncogenic potential: CHIP vs CHOP mutations in AML with NPM1 alteration. Leukemia. 2022;36(2):394–402. https://doi.org/10.1038/s41375-021-01368-1
30. Gondek LP. CHIP: is clonal hematopoiesis a surrogate for aging and other disease? Hematology Am Soc Hematol Educ Program. 2021;2021(1):384–9. https://doi.org/10.1182/hematology.2021000270
31. Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015;373(1):35–47. https://doi.org/10.1056/NEJMoa1414799
32. Babushok DV. A brief, but comprehensive, guide to clonal evolution in aplastic anemia. Hematology. Am Soc Hematol Educ Program. 2018;2018(1):457–66. https://doi.org/10.1182/asheducation-2018.1.457
33. Colden MA, Kumar S, Munkhbileg B, Babushok DV. Insights Into the Emergence of Paroxysmal Nocturnal Hemoglobinuria. Front Immunol. 2022;12:830172. https://doi.org/10.3389/fimmu.2021.830172
34. Robertson NA, Latorre-Crespo E, Terradas-Terradas M, LemosPortela J, Purcell AC, Livesey BJ, et al. Longitudinal dynamics of clonal hematopoiesis identifies gene-specific fitness effects. Nat Med. 2022;28(7):1439–46. https://doi.org/10.1038/s41591-022-01883-3
35. Bernstein N, Spencer Chapman M, Nyamondo K, Chen Z, Williams N, Mitchell E, et al. Analysis of somatic mutations in whole blood from 200,618 individuals identifies pervasive positive selection and novel drivers of clonal hematopoiesis. Nat Genet. 2024;56(6):1147–55. https://doi.org/10.1038/s41588-024-01755-1
36. Demajo S, Ramis-Zaldivar JE, Muinos F, Grau ML, Andrianova M, Lopez-Bigas N. Identification of Clonal Hematopoiesis Driver Mutations through In Silico Saturation Mutagenesis. Cancer Discov. 2024;14(9): 1717–31. https://doi.org/10.1158/2159-8290.CD-23-1416
37. Luis TC, Wilkinson AC, Beerman I, Jaiswal S, Shlush LI. Biological implications of clonal hematopoiesis. Exp Hematol. 2019;77:1–5. https://doi.org/10.1016/j.exphem.2019.08.004
38. Mooney L, Goodyear CS, Chandra T, Kirschner K, Copland M, Petrie MC, Lang NN. Clonal haematopoiesis of indeterminate potential: intersections between inflammation, vascular disease and heart failure. Clin Sci (Lond). 2021;135(7):991–1007. https://doi.org/10.1042/CS20200306
39. Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105. https://doi.org/10.1016/j.yjmcc.2021.07.004
40. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, et al. Association of Mutations Contributing to Clonal Hematopoiesis With Prognosis in Chronic Ischemic Heart Failure. JAMA Cardiol. 2019; 4(1):25–33. https://doi.org/10.1001/jamacardio.2018.3965
41. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111–21. https://doi.org/10.1056/NEJMoa1701719
42. Heyde A, Rohde D, McAlpine CS, Zhang S, Hoyer FF, Gerold JM, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell. 2021;184(5):1348–61.e22. https://doi.org/10.1016/j.cell.2021.01.049
43. Fuster JJ, Walsh K. Somatic Mutations and Clonal Hematopoiesis: Unexpected Potential New Drivers of Age-Related Cardiovascular Disease. Circ Res. 2018;122(3):523–32. https://doi.org/10.1161/CIRCRESAHA.117.312115
44. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1β/NLRP3 Inflammasome. J Am Coll Cardiol. 2018;71(8):875–86. https://doi.org/10.1016/j.jacc.2017.12.037
45. Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage Inflammation, Erythrophagocytosis, and Accelerated Atherosclerosis in Jak2 V617F Mice. Circ Res. 2018;123(11):e35–e47. https://doi.org/10.1161/CIRCRESAHA.118.313283
46. Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436):eaan8292. https://doi.org/10.1126/scitranslmed.aan8292
47. Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592(7853):296–301. https://doi.org/10.1038/s41586-021-03341-5
48. Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18(1):58–68. https://doi.org/10.1038/s41569-020-0431-7
49. Bouzid H, Belk JA, Jan M, Qi Y, Sarnowski C, Wirth S, et al. NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium. Clonal hematopoiesis is associated with protection from Alzheimer’s disease. Nat Med. 2023;29(7):1662–70. https://doi.org/10.1038/s41591-023-02397-2
50. Arends CM, Weiss M, Christen F, Eulenberg-Gustavus C, Rousselle A, Kettritz R, et al. Clonal hematopoiesis in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Haematologica. 2020;105(6):e264–e267. https://doi.org/10.3324/haematol.2019.223305
51. Hamada N, Fujimichi Y. Classification of radiation effects for dose limitation purposes: history, current situation and future prospects. J Radiat Res. 2014;55(4):629–40. https://doi.org/10.1093/jrr/rru019
52. Zhang Y, Chen X, Wang X, Chen J, Du C, Wang J, Liao W. Insights into ionizing radiation-induced bone marrow hematopoietic stem cell injury. Stem Cell Res Ther. 2024;15(1):222. https://doi.org/10.1186/s13287-024-03853-7
53. Jasra S, Giricz O, Zeig-Owens R, Pradhan K, Goldfarb DG, Barreto-Galvez A, et al. High burden of clonal hematopoiesis in first responders exposed to the World Trade Center disaster. Nat Med. 2022;28(3):468–71. https://doi.org/10.1038/s41591-022-01708-3
54. Yoshida K, French B, Yoshida N, Hida A, Ohishi W, Kusunoki Y. Radiation exposure and longitudinal changes in peripheral monocytes over 50 years: the Adult Health Study of atomicbomb survivors. Br J Haematol. 2019;185(1):107–15. https://doi.org/10.1111/bjh.15750
55. Brojakowska A, Kour A, Thel MC, Park E, Bisserier M, Garikipati VNS, et al. Retrospective analysis of somatic mutations and clonal hematopoiesis in astronauts. Commun Biol. 2022;5(1):828. https://doi.org/10.1038/s42003-022-03777-z
56. Koterov AN. From very low to very large doses of radiation: new data on ranges definitions and its experimental and epidemiological basing. Мedical radiology and radiation safety. 2013;58(2):5–21 (In Russ.). EDN: QEQHKM
57. Koterov AN, Ushenkova LN, Kalinina MV, Biryukov AP. “The healthy worker effect” in terms of overall mortality and mortality from malignant neoplasms among personnel of nuclear and chemical industry enterprises: meta-analyses. Мedical radiology and radiation safety. 2023;68(4):43–50 (In Russ.). https://doi.org/10.33266/1024-6177-2023-68-4-43-50
58. Koterov AN. Excess relative risk of mortality from diseases of the circulatory system after irradiation. Review and meta-analyses declaring the effects of low doses 2023. Мedical radiology and radiation safety. 2023;63(1):3–33 (In Russ.). https://doi.org/10.31857/S0869803123010095
About the Authors
A. A. ZherniakovaRussian Federation
Anastasiia Zherniakova
St. Petersburg
O. B. Krysiuk
Russian Federation
St. Petersburg
Ye. O. Kunevich
Russian Federation
St. Petersburg
Review
For citations:
Zherniakova A.A., Krysiuk O.B., Kunevich Ye.O. Clonal haematopoiesis and ionizing radiation: risks for hematological malignancies and somatic diseases. Extreme Medicine. 2024;26(4):5-12. https://doi.org/10.47183/mes.2024-26-4-5-12

















