Scroll to:
Soy lecithin-based liposomes for lymphatic delivery of biologically active substances
https://doi.org/10.47183/mes.2025-255
Abstract
Introduction. The targeted delivery of lipophilic chemotherapeutic and immunomodulatory agents through the lymphatic system is a promising approach in cancer treatment. Lipid-based carriers (e.g., liposomes) are able to not only to enhance the solubility and stability of drugs, but also to ensure their protection from degradation in the gastrointestinal tract. Research into the potential of lymphatic delivery of bioactive substances using liposomes can further improve the effectiveness of lipophilic drugs.
Objective. To study the prospects for using first-generation soy lecithin-based liposomes (cholesterol-free) as a lymphatic carrier for biologically active substances.
Materials and methods. Liposomes were prepared from soy lecithin containing green fluorescent protein GFP (with a maximum fluorescence at a wavelength of 506 nm) using the method of thin-film hydration/rehydration. Some liposomes were modified by 1%, 0.5%, and 0.1% chitosan solutions. The GFP incorporation into the liposomes was visualized using confocal microscopy. In vivo studies were conducted in three groups of female Balb/c mice aged 11–13 weeks (three animals in each): a control group; a group receiving native fluorescent protein, and a group with the design formulation (liposomes containing fluorescent protein). After intake, the small intestine was retrieved followed by its preparation and cryosection staining. The analysis of the cell suspension was performed using a CytoFLEX V5-B5-R3 flow cytometer.
Results. The confocal microscopy study found the particle size of the liposomes obtained by the thin-film hydration method to range within 1–5 μm. The incorporation of the model protein into the liposomes, as evidenced by its content before and after the liposome formation, was at least 60%. In vivo experiments on mice found that intragastric administration of fluorescent protein-containing liposomes enables successful protein delivery to the intestinal wall.
Conclusions. Soy lecithin-based liposomes were obtained using the thin-film hydration method. Confocal microscopy was used to evaluate the size of the obtained liposomes and to assess qualitatively the incorporation of green fluorescent protein. The incorporation of chitosan into the liposome shell resulted in a significant aggregation of the final product, which may reduce the effectiveness of liposome delivery to cells. Confocal microscopy of cryosections and cytofluorometry of cell suspensions obtained from small intestine fragments showed the capacity of the engineered system to deliver fluorescent protein and, possibly, intact liposomes to the intestinal wall.
Keywords
For citations:
Fedotova E.V., Skvortsov N.V., Perevoznikov I.E., Rogovskaya N.Yu., Beltyukov P.P., Bardin A.A., Babakov V.N., Krivorotov D.V., Radilov A.S. Soy lecithin-based liposomes for lymphatic delivery of biologically active substances. Extreme Medicine. 2025;27(3):320-327. https://doi.org/10.47183/mes.2025-255
INTRODUCTION
When ingested, many medicinal substances (MS) can be broken down by enzymes of the gastrointestinal tract (GIT), poorly absorbed in the small intestine, and are metabolized during the first passage through the liver. For such drugs, various lipid-based micro- and nanocarriers can be used to protect them from degradation under the influence of digestive juice components, to control release, to adjust distribution, and increase bioavailability, as well as for targeted drug delivery to the site of action [1]. Drug modification by conjugation with lipids (fatty acids, glycerides, and phospholipids) increases their lipophilicity and, consequently, their bioavailability [2]. In this case, however, it is necessary to examine whether the lipid-conjugated drug equals the original drug in terms of effectiveness. In some cases, it is possible to increase the drug bioavailability by surfactants. For example, sodium caprate can increase the bioavailability of certain drugs by increasing their permeability through the intestinal epithelium via paracellular transport [3].
Over the last few years, the possibility of developing lymphatic delivery systems for various drugs has been actively studied, bypassing the hepatic first-pass effect [4–6]. This route of administration can increase bioavailability. This strategy is particularly interesting for delivering antigens to lymph nodes and enhancing the adaptive immune response induced by vaccines. Conventional systemic chemotherapy requires high doses of drugs and often proves ineffective in delivering them to the lymphatic system. The development of carriers for drugs targeting lymphocytes is a unique opportunity to increase the effectiveness of HIV/AIDS therapy [7].
At present, a large number of different carriers are known that may be promising for lymphatic drug delivery (Fig. 1). In this case, the carrier must circulate in the blood for a long time and retain the drug until its accumulation in the target organ is reached [8–10]. In addition, the MS included in the carrier should not lose its activity during circulation. When selecting a drug carrier, it should be borne in mind that the effectiveness of internalization in a cell depends on its size, shape, and charge. For example, nanosized carriers make tighter contact with biological membranes than micron-sized carriers [9]. Polymer-based drug delivery systems are not always suitable for lymphatic delivery. Some charged polymers (such as chitosan) can bind to intestinal mucosal cells through non-covalent electrostatic interactions, hydrogen bonds, and Van der Waals forces [11].
Among all lipid-based nanocarriers, liposomes show the greatest promise due to their ability not only to encapsulate and protect drugs from degradation, but also to enhance their absorption in the intestine [4][12]. Liposomes are spherical vesicular delivery systems containing lipids, with a phospholipid bilayer located between two hydrophilic layers [13].
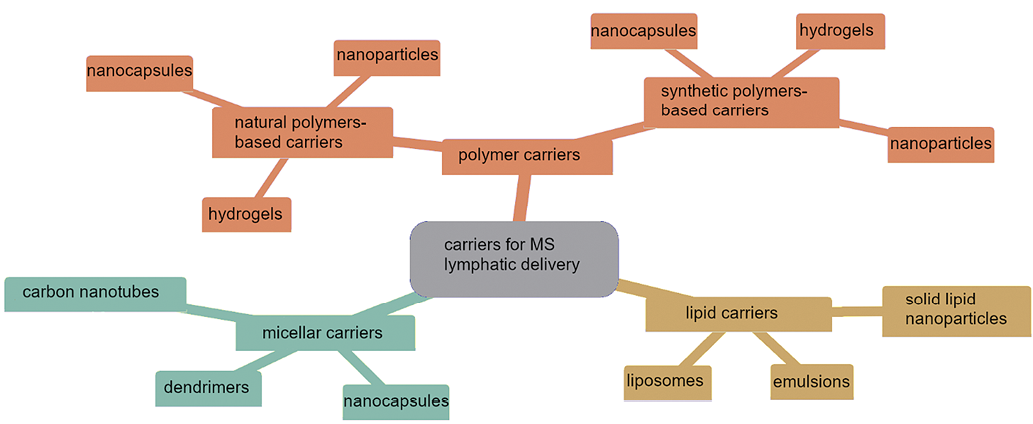
Figure prepared by the authors in the Mermaid graphical editor using data from [4–11]
Fig. 1. Promising carriers for lymphatic delivery of medicinal substances
The delivery mechanisms of macromolecules “packed” in liposomes may vary depending on the liposome size. Microparticles, including giant liposomes with linear dimensions similar to those of chylomicrons, are unable to penetrate blood capillaries, which have a pore size of approximately 60 nm. At the same time, the possibility of microparticles to pass through the mucous layer of the intestine has been experimentally confirmed in laboratory rodents for both biodegradable latex or polystyrene particles [14][15] and large liposomes up to 10 µm or greater [16]. One possible way for micro-sized particles to penetrate the intestinal barrier is their absorption through microfold M cells. Although the population of M cells among the total cell number is small (~1%), they capable of transcytosis of microparticles, bacteria, viruses, lipopolysaccharides, etc. This is due to the specific features of their structure and microenvironment (reduced density of the glycocalyx, less pronounced brush border and microvilli), which determines their ability to absorb microparticles larger than 200 nm, the maximum size of lipid complexes (micelles) that can be absorbed by enterocytes. It is believed that the main function of M cells is to deliver antigens for subsequent processing and presentation to a heterogeneous population of lymphocytes, macrophages, and dendritic cells that compose the gastrointestinal immune system. However, it is known that delivering cyclosporine A in large liposomes (~10 μm) via intragastric administration in rats results in a more than nine-fold increase in this drug bioavailability [16, 17]. It should be noted that, e.g., for protein antigens, this delivery route is the most promising, allowing the antigen to be delivered directly to the lymphoid tissue of the gastrointestinal (GI) tract. When large liposomes undergo transcytosis into the submucosal layer, some of them may enter other parts of the vascular system, including the systemic circulation, through the lymphatic vessels.
In this study, we explore the potential of using first-generation (cholesterol-free) soy lecithin-based liposomes as a carrier for potential lymphatic delivery of biologically active substances.
MATERIALS AND METHODS
In this work, soy lecithin (Ultralek P, ADM, USA), recombinant green fluorescent protein zFP506 (ZsGreen1), obtained in pAAV-ZsGreen1 Vector (Takara Bio) plasmid-transfected HEK293 cells (Thermo Fisher), hexane (analytic grade, Vecton, Russia), 0.05 M solution of sodium bicarbonate (bda, Vecton, Russia), 0.05 M solution of monosubstituted sodium phosphoric acid, 0.1 M hydrochloric acid (Vecton, Russia), 0.01 M phosphate-buffer saline (PBS) — pH 7.3 containing 0.137 M NaCl and 0.0027 M KCl (Biolot, Russia), Dulbecco’s solution without Ca and Mg (Biolot, Russia), bovine serum albumin (BSA) (Sigma-Aldrich), and 10% neutral formalin (Sintacon, Russia) were used.
Preparation of liposomes by thin-film hydration/rehydration
In a round-bottom flask, 100 mg of soy lecithin was dissolved in 20 mL of hexane (solvent). The resulting mixture was evaporated using a rotary evaporator (Heidolph, Germany) until a thin film was formed (water bath temperature of 45°C, pressure of 65 mbar). Green fluorescent protein (GFP) from the initial solution was diluted to a concentration of 5 mg/mL in 50 mL of 0.01 M sodium phosphate buffer (pH 7.4). The protein was incorporated into the liposomes passively during their formation from the resulting GFP solution in a flask, upon constant stirring on a magnetic stirrer (Heidolph, Germany) at 400 rpm (4 h at room temperature) until the film was fully rehydrated from the walls. The obtained liposomes were centrifuged in a centrifuge (Heidolph, Germany) at a rotor speed of 20,000 rpm for 15 min to precipitate the obtained liposomes, and the supernatant was collected and washed once with deionized water.
To assess the efficiency of protein incorporation into liposomes, the protein content was determined using the Lowry protein assay in accordance with OFS.1.7.2.0023.15 in the solution before and after the liposome formation. The protein concentration in the solution decreased by approximately 60% after the process was completed, compared to the initial concentration, which should correspond to the efficiency of GFP incorporation.
Preparation of chitosan solution
A chitosan solution at concentrations of 1%, 0.5%, and 0.1% was prepared by dissolving dry samples in a 1% acetic acid solution. The pH of the resulting solutions was monitored using a pH meter; and the pH was found to be 5.4.
Preparation of liposomes by thin-film hydration/rehydration with chitosan addition
In a round-bottomed flask, 100 mg of soy lecithin was dissolved in 20 mL of hexane. The resulting lecithin solution was evaporated using a rotary evaporator (Heidolph, Germany) until a thin film was formed (water bath temperature of 45°C, pressure of 65 mbar). A 5 mg/mL concentration of green fluorescent protein was dissolved in 50 mL of 0.01 M sodium phosphate buffer (pH 7.4) and injected together with chitosan solutions of selected concentrations. The formation of liposomes was carried out under constant stirring on a magnetic stirrer (Heidolph, Germany) at 400 rpm for 4 h at room temperature until the film was completely rehydrated from the walls. The resulting liposomes were centrifuged (Heidolph, Germany) at 20,000 rpm for 15 min; the supernatant was removed; and the liposomes were washed once with water.
Confocal microscopy
Confocal microscopy was performed (Zeiss LSM 710 microscope, CarlZeiss, Germany; magnification ×63) with an argon laser λ = 488 nm. In the studied samples, GFP with the maximum fluorescence at a wavelength of 506 nm was used as a model protein for imaging liposomes.
Purification of GFP and determination of its concentration
GFP was isolated from the cell extract of HEK293 cells transfected with the pAAV ZsGreen1 Vector plasmid (Takara Bio) using the method of alcohol extraction and ammonium sulfate precipitation described in [18].
In vivo studies
The work was carried out using female Balb/c mice, which were divided into three groups of three mice in each:
- group 1 — control group;
- group 2 — animals received native fluorescent protein;
- group 3 — animals received a suspension with the studied construct (liposomes without chitosan containing GFP).
The choice of this number of experimental animals in the groups is sufficient to assess the nature and frequency of the effects recorded. The animals were 11–13 weeks old at the beginning of the experiment, and their body weight did not deviate from the average value for all experimental groups by more than 20%.
The experimental mice were injected with GFP-containing liposomes without chitosan or an aqueous solution of GFP, as described above. The control group consisted of intact mice. In cell suspensions obtained from animals, the presence of GFP fluorescence was assessed using flow cytometry.
The intragastric injection procedure was performed by qualified specialists using a probe. The injection volume was the same for all animals, equaling 300 μL. The GFP concentration in the aqueous solution used for injection into mice was determined by the Lowry protein assay, comprising 0.86 mg/mL. The same GFP solution was used in the preparation of the liposomes. In the samples studied, the efficiency of GFP incorporation into liposomes was at least 60%.
Three hours after the administration of GFP solution or GFP-containing liposomes, the mice were euthanized by cervical dislocation. After opening the abdominal cavity, a 2-cm-long fragment of the small intestine was removed 1 cm from the stomach and transferred to a separate 5-mL tube containing PBS.
A 1-cm-long portion of each intestinal fragment was separated and cut lengthwise to create a rectangular preparation.
Preparation and staining of small intestine cryosections
Uncut 1 cm long small intestine fragments were washed in PBS, cut into 0.5 cm long fragments, and embedded in Tissue-Tek® O.C.T. Compound (Sakura Finetek, Japan), filling the gel from the inside to preserve the structure of the cryosections. The samples were frozen in liquid nitrogen and sliced using a cryotome (SLEE medical, Germany). 10-μm-thick cryosections were placed on a glass slide, washed twice with PBS, and fixed in neutral formalin (3.7%) for 30 min. The fixed samples were washed twice with PBS and permeabilized with 0.1% Triton X-100 solution in PBS with 1% BSA for 30 min, and then washed twice with PBS and 1% BSA.
To visualize cells by confocal microscopy, F-actin of the cellular cytoskeleton was stained with the Alexa Fluor 594 phalloidin fluorescent dye (Invitrogen, USA), diluted in PBS with 1% BSA in a ratio of 1:40, then 30 min and incubated in the dark. After that, the samples were washed twice with PBS and 1% BSA and enclosed in ProLong Gold antifade reagent (Invitrogen, USA). The drugs were stored in the dark at a temperature of 2–6 °C.
Flow cytometry
After washing the preparation from mucus and blood in a cuvette with PBS solution, it was transferred to a cell sieve with a 40 μm mesh size followed by addition of 1 mL of Dulbecco’s solution (Biolog). The cell suspension was then obtained by rubbing. The suspension was then washed twice with Dulbecco’s solution and centrifuged (8 min, 800 rpm). The washed cell pellet was resuspended in 400 μL of Dulbecco’s solution.
The cell suspension was analyzed using a CytoFLEX flow cytometer in the V5-B5-R3 configuration (Beckman Coulter, USA). For each sample, 50,000 events were collected (at least 20,000 for control samples) at a sample flow rate of 10 μL/s.
The number of GFP-containing cells was counted using standard forward and side scatter detectors (to determine the morphological characteristics of the cells, such as linear size and complexity of the intracellular structure, respectively), as well as a light filter suitable for GFP visualization (FITC channel).
RESULTS AND DISCUSSION
During the study, the liposome formation technique was selected based on the following criteria:
- Only gentle methods should be used to obtain the carrier (i.e., solvents that can alter the properties of the drug should not be used during the preparation of the delivery system; external factors such as temperature, ultrasound, high mixing rates, etc. should be minimized);
- The particle size of the delivery system should be less than 10 μm;
- The delivery system should contain biocompatible and non-toxic components;
- The resulting system, after oral administration, should ensure the delivery of the packaged drug to the submucosal layer of the intestine, possibly by transport through the lymphatic vessels.
Currently, the range of various modified liposomes has been significantly extended. It is believed that polymer coatings increase the ability of liposomes to cross the epithelial barrier. Chitosans and their derivatives are widely used for this purpose. When interacting with membrane proteins, chitosan can stimulate conformational changes in protein molecules, which can lead to increased paracellular transport of biologically active molecules. However, the application of hydrophilic polymers to liposomes may not always lead to a positive effect (e.g., protection against degradation of such liposomes by digestive enzymes is not always achieved). Liposomes containing chitosan are sensitive to changes in pH and can aggregate in the gastrointestinal tract, thus deteriorating drug absorption. This effect was demonstrated in the case of oral administration of cyclosporine A incorporated into chitosan-modified liposomes [19].
During the study, two variants of liposomes were formed: those with chitosan embedded in the liposomes (to increase the mucoadhesive properties) and those without modification. Several concentrations of the polymer (1%, 0.5%, and 0.1% chitosan solution in acetic acid) were tested in the study. It was found that the introduction of chitosan into the liposomes at all selected concentrations can lead to their strong aggregation (Fig. 2A), which significantly reduces their potential as a delivery system. The most logical solution to the aggregation problem is to apply ultrasonic treatment to the particles during their formation. However, according to the selected criteria for liposome formation, this treatment was not acceptable. Therefore, we selected a delivery system without any modifications for in vivo studies. The liposomes obtained without chitosan had a stable linear size of 1–5 μm (Fig. 2B) and showed no aggregation.
When analyzing the cytofluorometry results, the autofluorescence level in the FITC channel, which is always present in biological objects, was taken as the value corresponding to the control group. The latter was about 1% of the parent cell population in all the samples studied (Fig. 3A). In the samples of cell suspensions from mice in the group that received GFP without liposomes, the number of events corresponding to GFP fluorescence did not exceed the level of autofluorescence in the control (Fig. 3B).
In intestinal cell samples obtained from animals in the group that received GFP in liposomes, a significant increase in events corresponding to GFP fluorescence was observed, up to 8–10% of the parent cell population (Fig. 3C).
Confocal microscopy showed that GFP molecules packed in liposomes do not break down in the stomach and can be delivered to intestinal cells (Fig. 4B). The prospects for further use of the applied delivery system depend on the effectiveness of the MS packed in liposomes and its physical and chemical characteristics. In addition, it is planned to modify the liposomes by applying Pluronic 127 to their surface. This ligand is known to improve the liposome uptake by enterocytes and increase the liposome stability. Such modification can increase the delivery system absorption by cells and ensure efficient transport through the lymphatic system. When cyclosporine A was delivered orally by liposomes with Pluronic 127, the delivery system in the gastrointestinal tract demonstrated good stability. The selected carrier reached the epithelial surface, resulting in high drug absorption [19].
The efficiency of macromolecule delivery in liposomes by oral administration is still not considered satisfactory. Improving the delivery of liposomes to cells remains an important issue in current research [20]. In that study, the authors used nanosized liposomes containing cholesterol to deliver mRNA encoding GFP to Caco-2 cells, which are often used as a model for evaluating the delivery of liposomes by transcytosis. There is a lack of publications on the delivery of macromolecules in liposomes using in vivo biologics. The results obtained in our study appear important in terms of practical assessment of the liposome use for delivering macromolecules through the gastrointestinal tract.
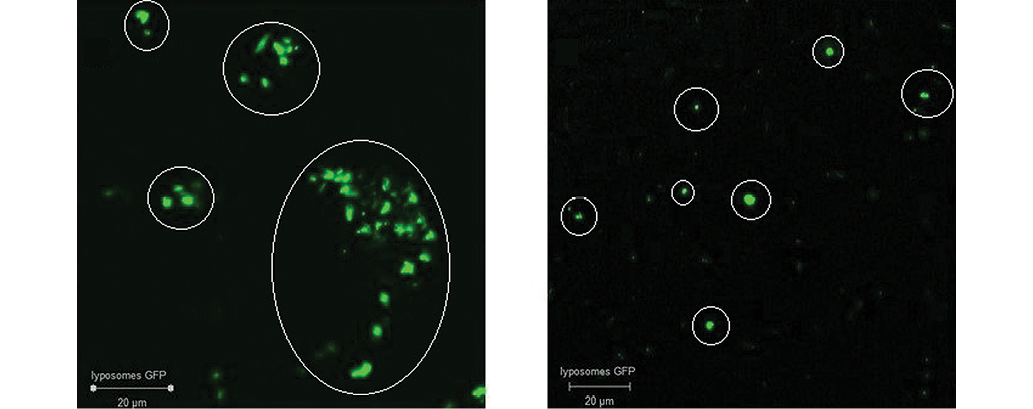
Photos taken by the authors
Fig. 2. Confocal micrographs of GFP-liposomes: A — with chitosan coating; B — selected methodology without modifications; mark — liposomes with GFP

Figure prepared by the authors using their own data
Fig. 3. Flow cytometry results for GFP-liposomes
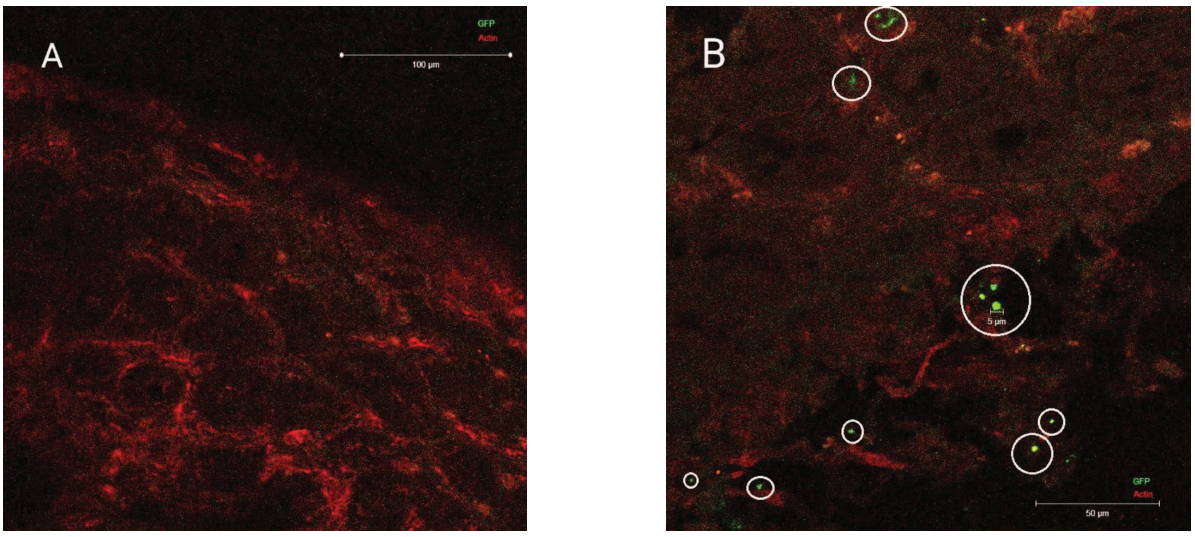
Photograph taken by the authors
Fig. 4. Results of confocal microscopy of the intestinal wall: А — after GFP injection without liposome packaging (no GFP complexes); В — intestinal wall after administration of GFP liposomes; mark — liposomes with GFP
CONCLUSIONS
- Soy lecithin-based liposomes were obtained by the thin-film hydration method.
- The size of the obtained liposomes was estimated by confocal microscopy, which confirmed the incorporation of green fluorescent protein. The liposomes obtained by the selected method showed an average size of 3–5 μm.
- It was shown that at the selected concentrations, chitosan application to liposomes can lead to significant aggregation of the final product, which in turn can negatively affect its further uptake by cells.
- The detection of liposomes in intestinal wall cross sections indicated the possibility of their transition to the submucosal layer and the absence of their complete destruction in the gastrointestinal tract.
- Flow cytometry confirmed the delivery of fluorescent protein to intestinal cells after intragastric administration of GFP in liposomes, which was evidenced by an increase in the proportion of cells with the GFP-corresponding fluorescence.
- In the intestine cryosections, confocal microscopy revealed the fluorescence areas similar in size to the liposomes used in the experiment. It is likely that some of the liposomes that are absorbed through transcytosis do not undergo biodegradation for at least 3 h after administration.
- It can be assumed that such liposomes can be used to deliver proteins or other macromolecules as antigens for immunization mediated by the cells of the gastrointestinal lymphoid tissue. However, this requires further confirmation.
References
1. McCright J, Naiknavare R, Yarmovsky J, Maisel K. Targeting lymphatics for nanoparticle drug delivery. Frontiers in Pharmacology. 2022;13:887402. https://doi.org/10.3389/fphar.2022.887402
2. Kim H, Kim Y, Lee J. Liposomal formulations for enhanced lymphatic drug delivery. Asian Journal of Pharmaceutical Sciences. 2013;8(2):96–103. https://doi.org/10.1016/j.ajps.2013.07.012
3. Rama A, Govindan I, Kailas AA, Annadurai T, Lewis SA, Pai SR, et al. Drug delivery to the lymphatic system: The road less travelled. Journal of Applied Pharmaceutical Science. 2024;14(6):1–10. https://doi.org/10.7324/JAPS.2024.180277
4. Ahn H, Park JH. Liposomal delivery systems for intestinal lymphatic drug transport. Biomaterials Research. 2016;20(1):36. https://doi.org/10.1186/s40824-016-0083-1
5. Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Advanced Drug Delivery Reviews. 2001;47(1):55–64. https://doi.org/10.1016/S0169-409X(00)00121-6
6. Yang Q, Forrest L. Drug delivery to the lymphatic system. Drug Delivery: Principles and Applications. 2016:503–48. https://doi.org/10.1002/9781118833322.ch21
7. Shao J, Kraft JC, Li B, Yu J, Freeling J, Koehn J, et al. Nanodrug formulations to enhance HIV drug exposure in lymphoid tissues and cells: clinical significance and potential impact on treatment and eradication of HIV/AIDS. Nanomedicine. 2016;11(5):545–64. https://doi.org/10.2217/nnm.16.1
8. Singh I, Swami R, Khan W, Sistla R. Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opinion on Drug Delivery. 2014;11(2):211–29. https://doi.org/10.1517/17425247.2014.866088
9. Cho HY, Lee YB. Nano-sized drug delivery systems for lymphatic delivery. Journal of Nanoscience and Nanotechnology. 2014;14(1):868–80. https://doi.org/10.1166/jnn.2014.9122
10. Porter CJ. Drug delivery to the lymphatic system. Critical Reviews in Therapeutic Drug Carrier Systems. 1997;14(4):333–94. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v14.i4.10
11. Managuli RS, Raut SY, Reddy MS, Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expert Opinion on Drug Delivery. 2018;15(8):787–804. https://doi.org/10.1080/17425247.2018.1503249
12. Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Advanced Drug Delivery Reviews. 2011;63(10–11):901–8. https://doi.org/10.1016/j.addr.2011.05.017
13. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: Structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. https://doi.org/10.1016/j.heliyon.2022.e09394
14. Hodges GM, Carr EA, Hazzard RA, Carr KE. Uptake and translocation of microparticles in small intestine. Digestive Diseases and Science. 1995;40:967–75. https://doi.org/10.1007/BF02064184
15. Donkers JM, Höppener EM, Grigoriev I, Will L, Melgert BN, van der Zaan B, et al. Advanced epithelial lung and gut barrier models demonstrate passage of microplastic particles. Microplastics and Nanoplastics. 2022;2(6):2–18. https://doi.org/10.1186/s43591-021-00024-w
16. Shah NM, Parikh J, Namdeo A, Subramanian N, Bhowmick S. Preparation, Characterization and In Vivo Studies of Proliposomes Containing Cyclosporine A. Journal of Nanoscience and Nanotechnology. 2006;6(9):2967–73. https://doi.org/10.1166/jnn.2006.403
17. Lee M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics. 2020;12(3):264. https://doi.org/10.3390/pharmaceutics12030264
18. Samarkina ON, Popova AG, Gvozdik EY, Chkalina AV, Zvyagin IV, Rylova YuV, et al. Universal and rapid method for purification of GFP-like proteins by the ethanol extraction. Protein Expression and Purification. 2009;65(1):108–13. https://doi.org/10.1016/j.pep.2008.11.008
19. Chen D, Xia D, Li X, Zhu Q, Yu H, Zhu C, et al. Comparative study of Pluronic® F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. International Journal of Pharmaceutics. 2013;449(1–2):1–9. https://doi.org/10.1016/j.ijpharm.2013.04.002
20. Dürr V, Wohlfart S, Eisenzapf T, Mier W, Fricker G, Uhl P. Oral Delivery of mRNA by Liposomes Functionalized with Cell-Penetrating Peptides. Applied Nano. 2023;4:293–308. https://doi.org/10.3390/applnano4040017
21.
About the Authors
E. V. FedotovaRussian Federation
Elena V. Fedotova, Cand. Sci. (Chem.)
Kuzmolovsky, Leningrad region
St. Petersburg
N. V. Skvortsov
Russian Federation
Nikita V. Skvortsov
Kuzmolovsky, Leningrad region
I. E. Perevoznikov
Russian Federation
Ilya E. Perevoznikov
Kuzmolovsky, Leningrad region
N. Yu. Rogovskaya
Russian Federation
Nadezhda Yu. Rogovskaya
Kuzmolovsky, Leningrad region
P. P. Beltyukov
Russian Federation
Petr P. Beltyukov, Cand. Sci. (Med.), Associate Professor
Kuzmolovsky, Leningrad region
A. A. Bardin
Russian Federation
Alexander A. Bardin
Kuzmolovsky, Leningrad region
V. N. Babakov
Russian Federation
Vladimir N. Babakov, Cand. Sci. (Biol.)
Kuzmolovsky, Leningrad region
D. V. Krivorotov
Russian Federation
Denis V. Krivorotov, Cand. Sci. (Chem.)
Kuzmolovsky, Leningrad region
A. S. Radilov
Russian Federation
Andrey S. Radilov, Dr. Sci. (Med.), Professor
Kuzmolovsky, Leningrad region
Supplementary files
Review
For citations:
Fedotova E.V., Skvortsov N.V., Perevoznikov I.E., Rogovskaya N.Yu., Beltyukov P.P., Bardin A.A., Babakov V.N., Krivorotov D.V., Radilov A.S. Soy lecithin-based liposomes for lymphatic delivery of biologically active substances. Extreme Medicine. 2025;27(3):320-327. https://doi.org/10.47183/mes.2025-255

















