Scroll to:
Determination of predictors of adverse disease outcome in patients with COVID-19 based on hemostasis system analysis
https://doi.org/10.47183/mes.2025-306
Abstract
Introduction. Severe complications of the novel coronavirus infection (COVID-19) include arterial or venous thromboses, which not only complicate the disease course but also increase mortality. The development of hypercoagulability, which precedes the occurrence of thrombosis, is associated with a significant activation of the hemostasis system, as well as the appearance of microparticles in circulation. These microparticles, generated by activated blood cells, enhance the procoagulant orientation of hemostasis. In this regard, assessment of the prognostic value of changes in hemostasis system parameters associated with the progression and outcome of COVID-19 represents a relevant research task.
Objective. To identify predictors of adverse outcomes of the novel coronavirus infection based on the assessment of parameters characterizing the state of the hemostasis system.
Materials and methods. A total of 163 patients (78 males and 85 females, aged 35–90 years, median age 69 years) were examined during the acute phase of the disease with severe and moderate severity. Depending on the disease outcome, the patients were divided into two groups: the group of survivors (n = 120) and the group of the deceased (n = 43). A study of plasma hemostasis parameters was conducted, including Quick’s prothrombin test, fibrinogen concentration, activated partial thromboplastin time, factor VIII activity, ristocetin cofactor activity, von Willebrand factor content, protein C activity, antithrombin, and free protein S. In addition, the characteristics of microparticles were studied. Statistical processing of the results was performed using the Statistica 12.0 software package.
Results. In patients with adverse disease outcomes, a significant decrease in Quick’s prothrombin time (PT) and antithrombin activity was observed, along with an increase in von Willebrand factor activity, D-dimer concentration, and platelet microparticle count. The analysis of sensitivity and specificity of these parameters allowed Quick’s PT less than 70% (sensitivity and specificity were 70% and 74.3%, respectively), D-dimer level more than 800 ng/ml (sensitivity and specificity — 72% and 75.2%, respectively), and platelet MP count more than 3.22% (sensitivity and specificity — 77.8% and 72.7%, respectively) to be considered as threshold values associated with lethal outcome from COVID-19.
Conclusions. Based on the conducted ROC analysis, predictive models for the risk of adverse outcomes of COVID-19 associated with changes in hemostasis system parameters were obtained. The parameters of D-dimer concentration, Quick’s prothrombin time, and platelet microparticle count can be used as laboratory predictors of unfavorable disease progression.
Keywords
For citations:
Matvienko O.U., Smirnova O.A., Golovina O.G. Determination of predictors of adverse disease outcome in patients with COVID-19 based on hemostasis system analysis. Extreme Medicine. 2025;27(4):587-593. https://doi.org/10.47183/mes.2025-306
INTRODUCTION
It has been established that the novel coronavirus infection (COVID-19) is associated with the development of endothelial dysfunction, activation of blood cells with the formation of blood plasma microparticles, plasma fibrinolytic failure, and the development of a cytokine storm syndrome [1][2]. Such pathological changes lead to a procoagulant shift in the hemostatic system, which may be associated with the development of hypercoagulability followed by thrombosis in the microvasculature, distress syndrome, and multiple organ failure. Thromboembolic complications are among the leading causes of increased mortality in patients with COVID-19.
Studies have shown that the frequency of thrombotic complications reaches 18% in patients treated in intensive care units [3][4]. During COVID-19, activation of the hemostatic system occurs, affecting both its plasma and cellular components, leading to a prothrombotic state [5–8]. Microparticles derived from various blood cells and capable of participating in a range of biological reactions may play a significant role in these procoagulant changes. Plasma microparticles are phospholipid microvesicles ranging 0.1–1 μm in size. They are surrounded by a cell membrane, lack a nucleus, and vary significantly in the composition of antigenic determinants on their surface, depending on the mechanism of their formation and the nature of the stimulating influence. Due to the negatively charged phospholipids and tissue factor localized on their surface, MPs actively participate in hemostatic reactions, which may be a significant factor in the development of thrombotic complications in various pathologies, including COVID-19 [9–11].
The use of various laboratory and instrumental studies to identify predictors of adverse outcomes and progression of the novel coronavirus infection is of great interest. Thus, determination of the SARS-CoV-2 viral load and assessment of lung CT results upon hospitalization using artificial intelligence have shown good prognostic value; however, these methods are not widely available for clinical use [12][13]. Certain informativeness is offered by the level of lymphopenia and changes in lymphocyte subpopulations, as well as such indicators as C-reactive protein, procalcitonin, and ferritin, which are nonspecific markers of inflammation [14–17]. Given the characteristic changes in the blood coagulation system accompanying COVID-19, the search for such markers is also conducted among hemostatic parameters. A number of researchers have noted a correlation between high D-dimer levels and mortality, although its threshold prognostic values vary widely [18–20].
The selection of parameters characterizing the state of plasma hemostasis and the degree of blood cell activation, which can be used for prognostic assessment of disease severity and outcome, will contribute to a more rational management of patients with COVID-19.
This study is aimed at identifying predictors of adverse outcomes of the novel coronavirus infection based on the assessment of parameters characterizing the state of the hemostasis system.
MATERIALS AND METHODS
The study group included 163 patients (78 males and 85 females, aged 35–90 years, median age 69 years) with severe or moderate forms of COVID-19. All the patients were treated in intensive care units. The inclusion criteria were the age over 18 years old and confirmed novel coronavirus infection with a positive laboratory test for SARS-CoV-2 RNA. The exclusion criteria were the age under 18 years, history or presence of oncological disease, HIV infection, hepatitis B and C, liver pathology with impaired function, kidney disease with altered glomerular filtration rate, and regular use of any anticoagulant drugs prior to the onset of the disease.
The severity of the disease was determined by the degree of lung involvement, which exceeded 25%, as well as patient comorbidities. Among the examined patients, 120 (74%) recovered, while 43 (26%) had an adverse (fatal) outcome. Depending on the disease outcome, patients were divided into two groups for the assessment of hemostasis parameters and microparticle (MP) characteristics: the group of survivors (n = 120) and the group of deceased patients (n = 43).
Blood samples for the study were collected upon patients’ admission to the hospital, prior to the initiation of specific therapy and anticoagulant prophylaxis, using a vacuum system with Vacutest vacuum tubes containing 3.2% sodium citrate as an anticoagulant.
The following parameters characterizing the state of plasma hemostasis were evaluated: Quick’s prothrombin time (PT), fibrinogen concentration (Fg), activated partial thromboplastin time (APTT), factor VIII activity (f.VIII), ristocetin cofactor activity and von Willebrand factor content (vWF:RCof and vWF:Ag, respectively), protein C activity (PC) and antithrombin (AT), as well as free protein S level (PS). Reagents from HemosIL (Instrumentation Laboratory, USA) were used, and all studies were performed in accordance with the manufacturer’s instructions for reagents and equipment. The listed parameters were determined using automated coagulometers of the ACL series, including ACL Top 300 CTS and ACL Elite Pro (Automated Coagulation Laboratory, Instrumentation Laboratory, USA).
For the analysis of microparticle characteristics using flow cytometry, platelet-poor plasma was centrifuged using a ThermoFisherScientific centrifuge (Germany) at 22°C for 30 min at 14,000 g. After high-speed centrifugation, the supernatant was aspirated, and the pellet was collected and resuspended by adding 100 μL of phosphate-buffered saline (PBS). The resulting microparticle suspension was used for further analysis. To determine the quantity and origin of microparticles, a laser flow cytometer Cytoflex (Beckman Coulter, USA) was employed using fluorescently labeled antibodies against surface markers of cells as follows: platelet-derived (CD41), leukocyte-derived (CD45), and endothelial-derived (CD144).
Statistical analysis of the results obtained was performed using the Statistica 12.0 software (StatSoft Inc., USA). Asymmetrical data distribution was identified using the Shapiro–Wilk test; the results are presented as median (Me) and interquartile [ 25–75 percentile] range [Q1–Q3]. Comparison between the two groups was conducted using the non-parametric Mann–Whitney U-test. To identify the threshold values of variables associated with adverse outcomes of COVID-19, ROC analysis was performed with the construction of ROC curves. The quantitative interpretation of ROC curves was assessed using the Area Under the Curve (AUC) metric, which represents the area bounded by the ROC curve and the axis of false positive rates. An AUC value below 0.5 indicated the unsuitability of the selected classification method, while an AUC above 0.7 characterized the high predictive power of the constructed model. The Youden index was used as a criterion for determining the optimal threshold point or cutoff value along the ROC curve, allowing evaluation of the difference between the proportion of true positive results (sensitivity) and the proportion of false positive results (specificity) to select the optimal threshold value. The critical level of statistical significance was set at 0.05.
RESULTS
The results obtained by comparing the plasma hemostasis parameters of the examined patients, depending on the disease outcome, are presented in Table 1.
The results presented in Table 1 indicate significant differences between the two groups of examined patients. Thus, an adverse disease outcome was associated with a 1.4-fold increase in von Willebrand factor activity (340.0 [ 260.1–420.0]; p = 0.01), a 1.2-fold decrease in Quick’s prothrombin time (65.3 [ 51.0–73.9]; p = 0.00), and a reduction in antithrombin activity to 85.3 [ 71.0–97.5]; p = 0.034 when compared to the group of survivors. The most significant differences involved the concentration of D-dimer, which was nearly four times higher in deceased patients (1670.0 [715.0–4334.5]; p ≤ 0.0001) relative to levels in patients with a favorable disease outcome. These findings are consistent with data obtained by other researchers who have also identified a significant increase in D-dimer levels as a marker of adverse progression and outcome of the novel coronavirus infection [21][22].
Additionally, we conducted an analysis of plasma microparticle characteristics in the examined patients based on disease outcome. The obtained results are presented in Table 2.
The data presented in Table 2 indicate a significant increase in platelet-derived microparticles in patients with adverse disease outcomes compared to survivors. No significant differences were observed between the two groups in the number of endothelial-derived MPs. Leukocyte-derived microparticles were detected in negligible quantities, which precluded robust statistical analysis.
For further analysis, results from tests with parameters that significantly differed between patient groups based on disease outcome were selected, namely:
- D-dimer concentration,
- von Willebrand factor (vWF) activity,
- antithrombin (AT) activity,
- Quick’s prothrombin time (PT),
- platelet-derived MP count.
ROC analysis of vWF activity and antithrombin activity did not yield high-quality predictive models for adverse outcomes of COVID-19. Conversely, ROC analysis for parameters such as D-dimer, Quick’s PT, and platelet-derived MP count — which are directly associated with adverse COVID-19 outcomes — demonstrated models with a high predictive power:
- AUC for D-dimer 0.787;
- AUC for Quick’s PT 0.747;
- AUC for platelet-derived MPs 0.798.
The results allowed the determination of threshold values for these parameters, indicating a high probability of lethal outcome. For D-dimer levels, the highest Youden index (47.2), corresponding to a sensitivity of 72% and specificity of 75.2%, was achieved at a threshold value of **800 ng/mL** (Fig. 1).
For the indicator of Quick’s prothrombin time (PT), the highest Youden index of 44.3, corresponding to a model sensitivity of 70% and specificity of 74.3%, was achieved at a threshold value of 70% (Fig. 2).
For the platelet-derived microparticle count, the highest Youden index of 50.5, corresponding to a sensitivity of 77.8% and specificity of 72.7%, was achieved at a threshold value of 3.22% of events (Fig. 3).
Thus, the analysis of sensitivity and specificity of the selected parameters allows us to consider the following markers as predictors of an adverse outcome in COVID-19:
- Quick’sprothrombin time (PT) below 70%,
- D-dimer level above 800 ng/mL,
- Platelet-derived microparticle (MP) count above 3.22% of events.
Table 1. Parameters characterizing the plasma hemostasis status of COVID-19 patients
|
Parameters |
Survivors (n = 120) |
Deceased (n = 43) |
Level of statistical significance*, p |
|
APTT |
0.88 [ 0.82–0.97] |
0.88 [ 0.82–1.08] |
0.407 |
|
PT, % |
79.0 [ 70.2–85.1] |
65.3 [ 51.0–73.9] |
0.000 |
|
FG, g/L |
5.4 [ 4.1–6.9] |
5.5 [ 3.6–8.0] |
0.931 |
|
F.VIII, % |
112.4 [ 85.8–165.5] |
150.0 [ 80.3–217.2] |
0.08 |
|
vWF:RCo, % |
250.5 [ 180.0–350.1] |
340.0 [ 260.1–420.0] |
0.01 |
|
vWF:Ag, % |
219.6 [ 198.3–321.2] |
340.0 [ 260.1–420.0] |
0.08 |
|
D-dimer, ng/mL |
387.0 [ 220.0–724.5] |
1670.0 [ 715.0–4334.5] |
<0.0001 |
|
АТ, % |
97.7 [ 84.3–105.0] |
85.3 [ 71.0–97.5] |
0.034 |
|
PC, % |
97.0 [ 79.7–117.3] |
88.0 [ 67.2–102.0] |
0.185 |
|
PS, % |
75.2 [ 56.5–90.0] |
62.7 [ 48.2–86.3] |
0.138 |
Table compiled by the authors based on original data
Note: * — comparison was performed between groups of COVID-19 patients; APTT — activated partial thromboplastin time, PT — Quick’s prothrombin time, FG — fibrinogen concentration, f.VIII — factor VIII activity, vWF:RCo — ristocetin cofactor activity, vWF:Ag — von Willebrand factor antigen, АТ — antithrombin activity, PC — protein C activity, PS — free protein S.
Table 2. Characterization of microparticles in COVID-19 patients
|
Cellular Marker |
Survivors (n = 15) |
Deceased (n = 9) |
Level of statistical significance*, p |
|
CD41+ (% of events) |
2.22 [ 1.385–3.25] |
4.27 [ 3.48–4.61] |
0.025 |
|
CD144+ (% of events) |
0.03 [ 0.02–0.03] |
0.05 [ 0.03–0.06] |
0.8 |
Table compiled by the authors based on original data
Note: * — comparison was performed between the study groups of COVID-19 patients.
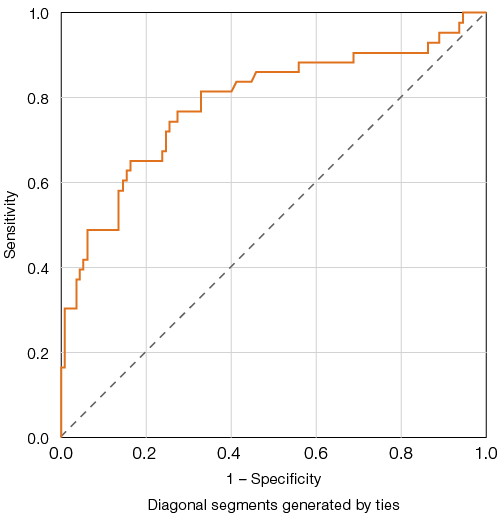
Figure prepared by the authors based on original data
Fig. 1. ROC curves for evaluating the predictive model of elevated D-dimer levels and adverse outcomes in COVID-19
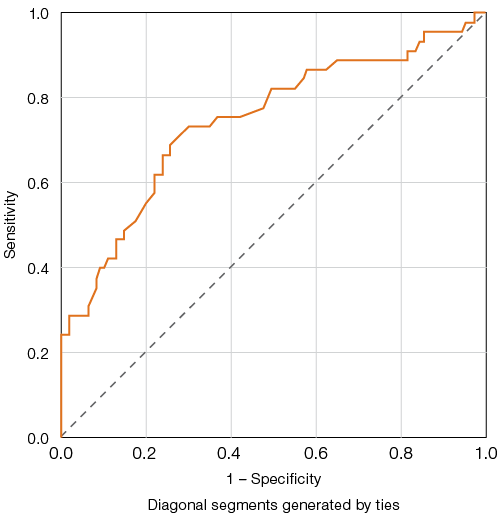
Figure prepared by the authors based on original data
Fig. 2. ROC curves for evaluating the predictive model of Quick’s prothrombin test (PT) reduction and adverse outcome in COVID-19
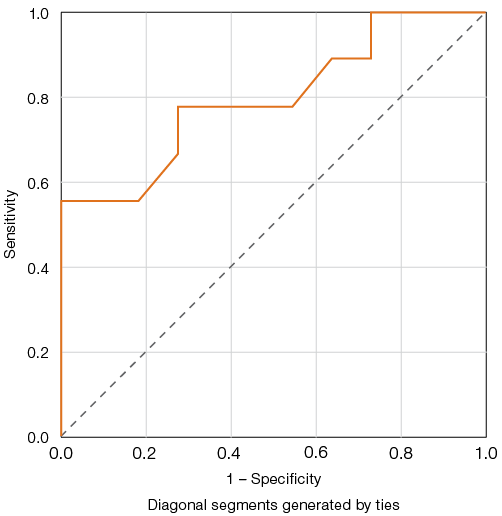
Figure prepared by the authors based on original data
Fig. 3. ROC curves for evaluating the predictive model of platelet-derived microparticles and adverse outcomes in COVID-19
CONCLUSION
Disturbances in the hemostatic system play a key role in the pathogenesis of COVID-19 complications. Prothrombotic changes lead to the development of thrombotic processes in vessels of various types and calibers, thus aggravating the disease prognosis. Our study revealed that a number of parameters characterizing the state of the hemostasis system, namely D-dimer concentration and Quick’s prothrombin time (PT), can be used as laboratory predictors of lethal disease outcomes. Given the limited size of the patient sample in which microparticle characteristics were determined, further research is needed to establish their significance for the development of adverse COVID-19 progression and outcomes.
References
1. Galstyan GM. Coagulopathy in COVID-19. Pulmonology. 2020;30(5):645–57 (In Russ.). https://doi.org/10.18093/0869-0189-2020-30-5-645-657
2. Bulanov AYu, Roitman EV. New coronavirus infection, hemostatic and heparin dosing problems: it is important to say now. Thrombosis, Hemostasis and Rheology. 2020;2:11–8 (In Russ.). https://doi.org/10.25555/THR.2020.2.0913
3. Lobastov KV, Porembskaya OYa, Schastlivtsev IV. Efficiency and safety of antithrombotic therapy in COVID-19. Ambulatory Surgery. 2021;18(2):17–30 (In Russ.). https://doi.org/10.21518/1995-1477-2021-18-2
4. Di Minno AD, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Seminars in Thrombosis and Hemostasis. 2020;46(7):763–71. https://doi.org/10.1055/s-0040-1715456
5. Matvienko OYu, Korsakova NE, Lerner AA, Shvedova TN, Papayan LP. The state of the plasma hemostasis in patients with coronavirus infection caused by the SARS-CoV-2 virus. Thrombosis, Hemostasis and Rheology. 2020;4:52–6 (In Russ.). https://doi.org/10.25555/THR.2020.4.0945
6. Schulman S, Hu Y, Konstantinides S. Venous thromboembolism in COVID-19. Thrombosis and Haemostasis. 2020;120(12):1642–53. https://doi.org/10.1055/s-0040-1718532
7. Kuznetsov SI, Shestakov EA, Zhiburt EB. Coagulopathy in COVID-19 infection. Thrombosis, Hemostasis and Rheology. 2020;4:31–4 (In Russ.). https://doi.org/10.25555/THR.2020.4.0942
8. Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Journal of Thrombosis and Haemostasis. 2020;18(7):1747–51. https://doi.org/10.1111/jth.14854
9. Momot AP, Tsarigorodtseva NO, Fedorov DV, Bishevski KM, Vostrikova NV, Klimova EE. Platelet microvesicles and their role in providing hemostatic potential (literature review). Siberian Scientific Medical Journal. 2020;40(2):4–14 (In Russ.). https://doi.org/10.15372/SSMJ20200201
10. Zubairov DM, Zubairova LD. Microvesicles in the blood. Functions and their role in thrombus formation. Moscow: GEOTAR-Media; 2009 (In Russ.).
11. Sirotkina OV, Ermakov AI, Gaykovaya LB, Kudlay DA, Vavilova TV. Microparticles of blood cells in patients with COVID-19 as a marker of hemostasis activation. Thrombosis, Hemostasis and Rheology. 2020;4:35–40 (In Russ.). https://doi.org/10.25555/THR.2020.4.0943
12. Tang K, Wu L, Luo Y, Gong B. Quantitative assessment of SARS-CoV-2 RNAemia and outcome in patients with coronavirus disease. Journal of Medical Virology. 2021;93(5):3165–75. https://doi.org/10.1002/jmv.26876
13. Li Y, Shang K, Bian W, He L, Fan Y, Ren T, et al. Prediction of disease progression in patients with COVID-19 by artificial intelligence assisted lesion quantification. Scientific Reports. 2020;10(1):22083. https://doi.org/10.1038/s41598-020-79097-1
14. Ozel AS, Altunal LN, Aydin M, Unal M, Cam G, Ozer MC, et al. Clinical characteristics and risk factors associated with severe disease and outcome of patients with COVID-19. Journal of Infection in Developing Countries. 2022;16(3):435–44. https://doi.org/10.3855/jidc.15411
15. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine. 2020;58(7):1021–8. https://doi.org/10.1515/cclm-2020-0369
16. Mishchenko TA, Ermakova PA, Ermakova AA, Tseller LP, Rogozhkina YA, Verteletskaya MI, et al. Predictors of severe course of new coronavirus infection (COVID-19): study design. Therapeutic Archive. 2022;94(11):1246–51 (In Russ.). https://doi.org/10.26442/00403660.2022.11.201402
17. Bobcakova A, Petriskova J, Vysehradsky R, Kocan I, Kapustova L, Barnova M, et al. Immune profile in patients with COVID-19: lymphocytes exhaustion markers in relationship to clinical outcome. Frontiers in Cellular and Infection Microbiology. 2021;11:646688. https://doi.org/10.3389/fcimb.2021.646688
18. Rajpoot A, Mishra M, Banerjee S, Kumar A, Panda PK, Sindhwani G, et al. Predictors of poor outcome in patients with COVID-19 associated respiratory failure: a retrospective observational study. Journal of the Association of Physicians of India. 2023;71(4):11–2.
19. Oudkerk M, Büller HR, Kuijpers D, Oudkerk SF, van Beek JR. D-Dimer and COVID-19. Radiology. 2020;297(3):343–4. https://doi.org/10.1148/radiol.2020203481
20. Zhang L, Yan X, Fan Q, Liu X, Liu Z, Zhang Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. Journal of Thrombosis and Haemostasis. 2020;18(6):1324–9. https://doi.org/10.1111/jth.14859
21. Momot DA, Mamaev AN, Nikolaeva MG, Momot AP, Kudinov AV, Neimark MI. Advancing plasma D-dimer level determination: an applicability analysis for COVID-19 patients at the hospital stage. Bulletin of Medical Science. 2023;2(30):79–86 (In Russ.). https://doi.org/10.31684/25418475-2023-2-79
22. Vorobyeva NA, Vorobyeva AI. Predictive value of D-dimer in COVID-19. Health Care Standardization Problems. 2021;5– 6:36–42. https://doi.org/10.26347/1607-2502202105-06036-042
About the Authors
O. U. MatvienkoRussian Federation
Olesia U. Matvienko
St. Petersburg
O. A. Smirnova
Russian Federation
Olga A. Smirnova
St. Petersburg
O. G. Golovina
Russian Federation
Olga G. Golovina
St. Petersburg
Supplementary files
Review
For citations:
Matvienko O.U., Smirnova O.A., Golovina O.G. Determination of predictors of adverse disease outcome in patients with COVID-19 based on hemostasis system analysis. Extreme Medicine. 2025;27(4):587-593. https://doi.org/10.47183/mes.2025-306

















