Scroll to:
The effect of general hyperthermia and local cooling on fentanyl tolerance in rats
https://doi.org/10.47183/mes.2025-311
Abstract
Introduction. The toxicity of a number of xenobiotics increases with air temperature. However, it remains unknown whether this applies to narcotic analgesics and whether this dependence can be corrected by first aid measures recommended for heat stroke.
Objective. Evaluation of the effect of elevated air temperatures and local cooling on the acute toxicity of fentanyl.
Materials and methods. Three series of experiments were conducted. In the first series, the effect of elevated air temperatures on the dose dependence of the lethal and narcotic effects of fentanyl was studied. In total, 11 groups of 20 rats each were formed, which were intravenously administered fentanyl at doses of 50, 100, 200, 300, or 400 µg/kg, and one group (n = 14) without drug administration. Following fentanyl administration, one subset of rats (n = 100) was kept for 24 h at an air temperature of 22 °C; the second subset (n = 100) was kept for 40 min in a thermal chamber at 40 °C and then for 24 h at 22 °C. Those not receiving fentanyl were observed in a thermal chamber until the first case of death, then for 24 h at 22 °С. In the second series of experiments, the effect of head cooling on lethality, latent awakening time, and rectal temperature of rats (n = 49) 40 min after intravenous administration of fentanyl at a dose of 300 µg/kg (LD5) was studied. Four groups of animals were formed, which were kept after fentanyl administration for 40 min at 22 or 40 °С with or without local cooling of the neurocranium, followed by observation for 24 h at 22 °С. In the third series of experiments, following the same scheme, the effect of cooling the middle third of the ventral surface of the torso on lethality, latent awakening time, and rectal temperature of rats (n = 48) 40 min after fentanyl administration at the same dose was studied. Statistical analysis was performed using the OriginPro software.
Results. A 40-min exposure at 40 °С was non-lethal for intact rats. After administration of fentanyl at doses of 100–400 µg/kg, lethality reached 0–5% and 60–95% at 22 °С and 40 °С, respectively. Hyperthermia induced by 40 °С exposure under fentanyl administration at a dose of 300 µg/kg was mitigated by head cooling and prevented by cooling the ventral surface of the torso. Cooling the ventral surface of the torso, rather than the head, reduced lethality from 100% to 8%. At 22 °С, both local cooling methods deepened fentanyl-induced hypothermia without significantly affecting lethality or anesthesia duration.
Conclusions. The general overheating potentiates the lethal and narcotic effects of fentanyl in rats. Under these conditions, cooling the ventral surface of the torso is an effective measure to prevent hyperthermia and lethality, while head cooling is ineffective. At room temperature, both local cooling methods deepen fentanyl-induced hypothermia without significantly affecting lethality. The efficacy of cooling the ventral surface of the torso requires evaluation not only during combined overheating but also during isolated overheating of the organism.
Keywords
For citations:
Ivnitsky J.J., Vakunenkova O.A., Golovko A.I., Lapina N.V., Rejniuk V.L. The effect of general hyperthermia and local cooling on fentanyl tolerance in rats. Extreme Medicine. 2025;27(4):475-482. https://doi.org/10.47183/mes.2025-311
INTRODUCTION
Overheating of the body is a health condition caused by an increase in body temperature under the conditions where the sum of the thermal energy released during metabolic processes and received from the environment exceeds the thermal energy lost through radiation, convection, and heat conduction.
Climatic conditions conducive to overheating of the body are not uncommon in Russia. One aspect of this problem is the associated increase in the toxicity of a number of xenobiotics for poikilothermic [1] and homeothermic animals [2]. In this regard, the influence of conditions promoting overheating of the body on the severity of side effects of narcotic analgesics is of interest. Their overdose may occur during the medical evacuation of the wounded. In addition, fentanyl derivatives are spreading rapidly on the illegal market of narcotic drugs and psychotropic substances [3]. However, there is a lack of data on predicting the effect of overheating on the toxicity of opioids. In order to fill this gap, the toxicity of such substances in cases of overdose under controlled climatic conditions should be studied.
Opioid overdose can be conveniently simulated by administering fentanyl to animals, due to a combination of short duration of action and its membership in the class of extremely toxic substances [4]. In addition, it is of interest to study the feasibility of therapeutic measures recommended for isolated overheating of the body in the form of heat stroke in the setting of opioid analgesia. The Russian Standard of Emergency Medical Care for Heat and Sun Stroke1 prescribes the “application of an ice pack” as such a measure, although not specifying the location for its placement. In educational and methodological sources, the forehead is most often indicated as such a location2. The back of the head, neck, temples, collarbones, inner bends of the elbow and knee joints, calf muscles, groin, and sacral areas are also mentioned [5]; however, there is a lack of objective data characterizing the validity of such recommendations.
This study aims to evaluate the effect of elevated air temperature and local cooling on the acute toxicity of fentanyl.
MATERIALS AND METHODS
The study was conducted in November 2024 using male outbred albino rats weighing 191–210 g from the Rappolovo Laboratory Animal Breeding Facility of the Kurchatov Institute. Three series of experiments were performed.
In the first series of experiments, the effect of elevated air temperature on the dose dependence of the lethal and narcotic effects of fentanyl was studied. In total, 11 randomized groups were formed. In 10 groups, animals (n = 200) were injected with a fentanyl solution of 50 µg/mL (Moscow Endocrine Plant, batch 30212) at doses of 50, 100, 200, 300, 400 µg/kg into the lateral tail vein. In one group (n = 14), the drug was not administered.
The individuals that received fentanyl (n = 100) were left for 24 h at room temperature of 22 °C (control group); the second part of the animals (n = 100) was placed for 40 min in open enclosures installed in a thermal chamber at 40 °C, and then observed for 24 h at an air temperature of 22 °C.
Rats that did not receive fentanyl (dose 0 mg/kg, volume 0 mL/kg) were placed in restrainers and kept in a thermal chamber until the first case of death. After removal from the thermal chamber, observation continued for 24 h at 22 °C. During this time, awakening (at the moment of the first running movement upon tail percussion) or death was recorded. The study design is presented in the Table.
In the second series of experiments using other rats (n = 49), the effect of general overheating and/or local head cooling on rectal temperature, lethality, and the latent time of awakening 40 min after the administration of the officinal fentanyl preparation at a dose of 300 µg/kg was studied. This dose was preliminarily identified as LD5 for individuals kept at room temperature. Four randomized groups were formed:
- Group 1 (n= 14) — animals that received fentanyl at a dose of 300 µg/kg and were then kept for 24 h at 22 °C without local cooling;
- Group 2 (n= 12) — animals that received fentanyl at a dose of 300 µg/kg, then left for 40 min at 22 °C with local cooling, and then for 24 h at 22 °C without local cooling;
- Group 3 (n= 12) — animals that received fentanyl at a dose of 300 µg/kg, kept for 40 min at 40 °C without local cooling, and then for 24 h at 22 °C;
- Group 4 (n= 11) — animals that received fentanyl at a dose of 300 µg/kg, kept for 40 min at 40 °C with local cooling, and then for 24 h at 22 °C.
To cool the head, plastic containers containing 70 g of melting ice were held on the surface of the neurocranium of the animals for 40 min after fentanyl administration, after which local cooling was stopped and body temperature was measured. The outcome of intoxication (death or awakening) was recorded over 24 h.
According to the same scheme, in the third series of experiments using four groups of 12 rats each, the influence of a 40-min stay in a thermal chamber at 40 °C and/or cooling the middle third of the ventral surface of the torso on acute fentanyl intoxication was studied. The conditions of local body cooling are illustrated in Fig. 1.
Climatic conditions promoting body overheating were simulated in a BMT Stericell SC 111 ECO thermal chamber with a capacity of 111 L (Czech Republic), which maintained an air temperature of 40 ± 1 °C, relative humidity of 48%, and an air exchange rate of 45 chamber volumes per hour. Body temperature was measured with an electric thermometer equipped with a RET-2 rat sensor (WPI, China), the tip of which was inserted into the rectum to a depth of 3 cm. Instrumental ophthalmoscopy was not performed; the color of the ocular fundus was indirectly assessed by the color of the eyes, which in albino rats lack pigments other than hemoglobin in the blood vessels.
Statistical analysis was performed using the OriginPro software. The results are presented as the mean value and its error (M ± m). The Shapiro–Wilk test was used to check the normality of the distribution. The influence of the applied interventions on parametric indicators was assessed using analysis of variance. In cases of significant models, intergroup comparison of means was performed using Tukey’s honestly significant difference test. LD50 values were calculated using the probit method. Intergroup differences in survival functions were assessed using the Gehan–Wilcoxon test, and the frequency of occurrence of alternative traits was assessed using Fisher’s exact method. The critical significance level α was set at 0.05.
RESULTS
In the first series of experiments, when studying the dose dependence of the lethal and narcotic effects of fentanyl, 10–15 s after its administration, opisthotonus, tail extension, and short-term apnea followed by rare shallow breathing were observed in the rats. The ocular fundus acquired a violet color. All cases of death occurred within the first 2 h, and awakenings occurred within the first 3.4 h. For individuals kept at room temperature, fentanyl doses of 50 and 100 µg/kg were sublethal, while at doses of 200, 300, or 400 µg/kg, the lethality rate was 5%. Thus, at room air temperature, the LD50 value for fentanyl administered intravenously as an official solution in a volume acceptable for rats (≤ 2 mL) was not reached.
In animals placed in the thermal chamber for 40 min after fentanyl administration, the LD50 was 113 ± 16 µg/kg (Fig. 2A). Thermal exposure against the background of fentanyl administration increased the latent time to awakening by 1.6–4.0 times (from 10–110 to 40–176 min, Fig. 2B). In such animals, transient ataxia was observed after awakening, which was absent in the control group. For rats that did not receive fentanyl, the 40-min thermal exposure was non-lethal; the first case of death was noted only 90 min after being placed in the thermal chamber.
Figure 2 shows that, compared to animals left at room temperature, the difference is significant in the dose range of 100–400 µg/kg for lethality and in the dose range of 50–400 µg/kg for the latent time of awakening, p < 0.05.
In the second and third series of experiments, when studying the thermal state of the body, the initial rectal temperature was 38.1 ± 0.1 °C (n = 96). Thus, 40 min after fentanyl administration, the rectal temperature of surviving individuals in Group 1, left at room temperature without local body cooling, decreased to 34.5 ± 0.2 °C (n = 25), while in rats from Group 3 (n = 5) that remained alive at this time, it increased to 44.5 ± 0.4 °C. With head cooling, the body temperature in rats left at room temperature was 33.7 ± 0.3 °C, Group 2 (n = 10), and in the six surviving individuals from Group 4 at the time of removal from the thermal chamber, it was 39.8 ± 0.6 °C (Fig. 3A). Cooling the ventral surface of the torso reduced the body temperature of animals compared to those not receiving local cooling in animals that, after fentanyl administration, were kept at both room and elevated air temperatures: to 29.7 ± 0.3 °C (n = 9) and 33.0 ± 0.5 °C (n = 11) over 40 min, respectively. Over 40 min, the difference in mean group body temperature values among the rats that survived exposure to the thermal chamber, without and with local cooling of the ventral torso surface, reached 11.4 °C (Fig. 3B).
In rats left at room temperature after fentanyl administration, lethality was 7–8%, while in those placed in a thermal chamber for 40 min, it was 100% (p < 0.05). The effect of head cooling on lethality showed a tendency to increase by 10% in rats left at room temperature and to decrease by 27% in those placed in the thermal chamber (Fig. 4A).
Cooling the ventral surface of the torso completely prevented the aggravating effect of elevated air temperature on acute intoxication. Thus, the lethality decreased by 92% and was similar to that in rats left at room temperature without local cooling (Fig. 4B). In animals left at room temperature after fentanyl administration, cooling the ventral surface of the torso showed a tendency to increase lethality by 17% compared to those without local cooling. Rats subjected to thermal exposure after fentanyl administration without local body cooling died without awakening. No significant intergroup differences in the latent time of awakening were recorded among the remaining animals, who did not show signs of ataxia.
Table. Scheme for studying the influence of conditions promoting body overheating on the dose dependence of the lethal and narcotic effects of fentanyl
|
Rat number |
Pharmacological effects |
Climate impact |
|
|
Fentanyl dose, μg/kg |
Drug volume, mL/kg |
||
|
20 |
50 |
1 |
22 °C within 24 h after fentanyl administration |
|
20 |
100 |
2 |
|
|
20 |
200 |
4 |
|
|
20 |
300 |
6 |
|
|
20 |
400 |
8 |
|
|
14 |
0 |
0 |
40 °C until the occurrence of first death, and then 22 °C for 24 h |
|
20 |
50 |
1 |
40 °C for 40 min after the fentanyl administration and then for 24 h at 22 °C |
|
20 |
100 |
2 |
|
|
20 |
200 |
4 |
|
|
20 |
300 |
6 |
|
|
20 |
400 |
8 |
|
Table prepared by the authors
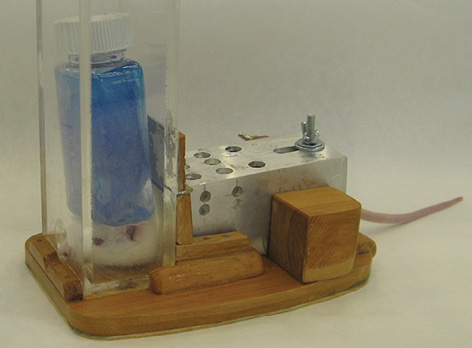
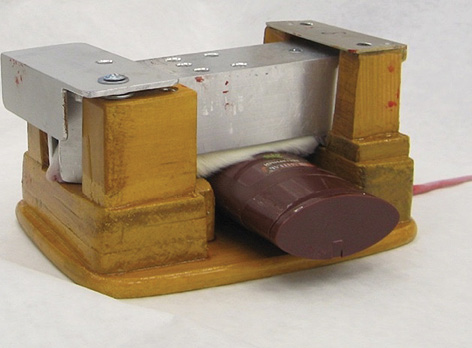
Figure prepared by the authors
Fig. 1. Local cooling of the rat body after fentanyl administration: A — container with melting ice is in contact with the neurocranium; B — container with melting ice is in contact with the middle third of the ventral surface of the torso
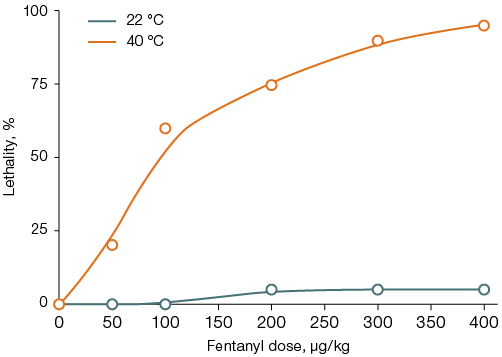
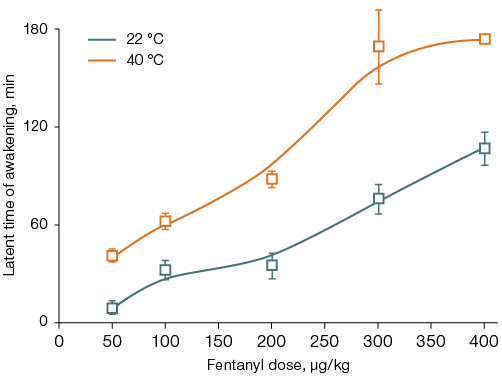
Figure was prepared by the authors
Fig. 2. Lethality (A) and latent time of awakening (B) in rats after fentanyl administration followed by a 40-min stay at an air temperature of 22 °C or 40 °C
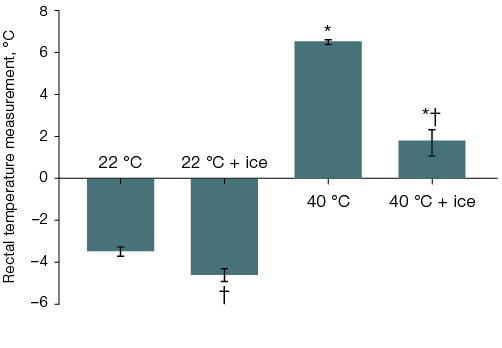
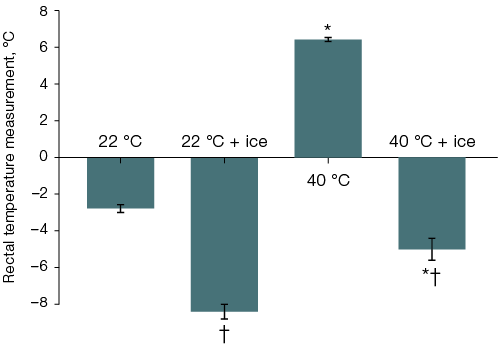
Figure prepared by the authors
Fig. 3. Changes in the rectal temperature of rats after fentanyl administration at a dose of 300 µg/kg and a 40-min stay at an air temperature of 22 °C or 40 °C with ice cooling of the head (A) or the ventral surface of the torso (B)
Note: statistically significant difference (p < 0.05): * — compared to the corresponding group of rats left at room temperature after fentanyl administration; † — compared to the corresponding group of rats without local cooling.
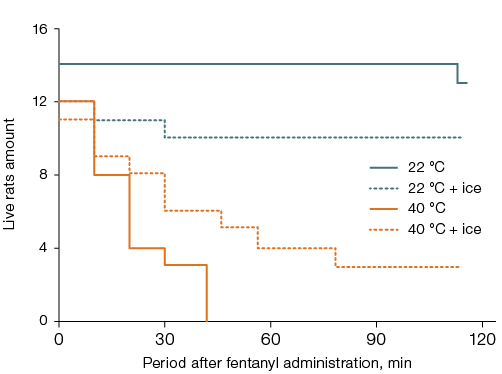
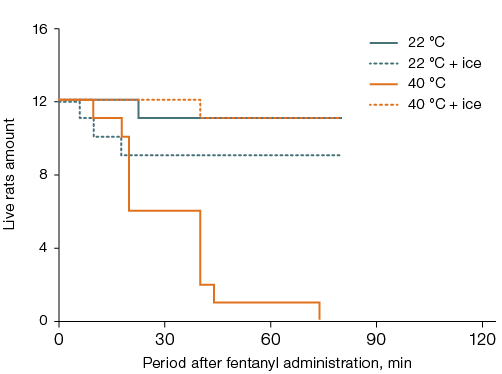
Figure prepared by the authors
Fig. 4. Survival of rats after fentanyl administration at a dose of 300 µg/kg and a 40-min stay at an air temperature of 22 °C or 40 °C with ice cooling of the head (A) or the ventral surface of the torso (B)
Note: statistically significant effect (p < 0.05) was found for: general overheating without local cooling (A, B); local cooling during general overheating (B).
DISCUSSION
The range of fentanyl doses used in the present study, 50–400 µg/kg, included the ED100 for analgesic activity in rats: 75 µg/kg [6]. When calculated based on body surface area, the administered doses are bioequivalent to 8–67 µg/kg for humans. This is 1–8 times higher than the doses used for neuroleptanalgesia and 2–16 times higher than the doses used in surgeries with spontaneous breathing3, modelling an overdose of an opioid narcotic analgesic.
While unlikely in a hospital setting, such an overdose is possible due to lapses in the continuity of care for the wounded during stages of medical evacuation, including under conditions promoting body overheating. In humans performing light physical work, overheating occurs when the air temperature exceeds 31 °C as measured by a “wet” bulb thermometer [7], which, at a relative humidity of 48%, corresponds to 40 °C on a “dry” bulb thermometer. According to the data obtained, a 40-min exposure to such conditions was easily tolerated by intact rats but severely exacerbated the toxic effects of opioids at doses that are non-lethal under thermal comfort conditions. These data characterize the type of interaction between the undesirable side effects of the opioid and conditions promoting body overheating as potentiation.
Overheating in animals placed in a thermal chamber after fentanyl administration occurred despite its hypothermic effect observed at room temperature [8]. In the setting of fentanyl exposure, overheating may have been facilitated by impaired evaporative heat loss from the body surface, which is hypothetically achieved in rats by spreading saliva onto their fur [9]. Narcotization deprived the animals of this thermoregulatory mechanism, analogous to sweat evaporation in humans. Reduced evaporation of moisture from the respiratory tract surface due to suppressed external respiration may also have contributed to decreased heat loss.
Exposure to a thermal chamber increased the rectal temperature of anesthetized rats to 44.5 °C, exceeding the threshold for irreversible tissue damage (43 °C) by 1.5 °C [10]. This was a sufficient condition for their death. In comparison, without thermal exposure, the administration of an equivalent dose of fentanyl was non-lethal for most animals. Reversible impairments, such as increased blood–brain barrier permeability and elevated brain water content, can occur even at lower body temperatures (42 °C) [10]; these may have been associated with the ataxia observed in rats that awakened after fentanyl administration and thermal chamber exposure. Thus, in the absence of local body cooling, hyperpyrexia was the factor precluding survival at an air temperature of 40 °C during acute fentanyl intoxication.
When the ventral torso surface or head was cooled, the body temperature did not reach 42 °C during the stay of animals in a thermal chamber. Although head cooling prevented hyperpyrexia during thermal chamber exposure, it did not significantly affect lethality. Probable mechanisms of thanatogenesis in these rats included cerebral edema and swelling, which have been described in both severe opioid intoxications [11] and heat stroke [12]. An increase in brain volume within the confined space of the skull leads to elevated intracranial pressure and reduced cerebral blood flow velocity. This may explain the lower cooling efficacy of ice application to the head compared to the ventral torso surface. These findings appear to contradict reports of high cerebral blood flow velocity4, high heat flux density from the head surface [13], and recommendations to cool the head during body overheating [5][14]. This contradiction may be explained by the hypothesis of reduced cerebral blood flow velocity, which impeded heat transfer from the body thermal “core” to the cooled surface of the neurocranium during acute fentanyl intoxication under conditions of body overheating.
Cooling the middle third of the ventral surface of the torso proved to be a highly effective measure for preventing body overheating despite elevated air temperatures and reduced evaporative heat loss. This indicates the sufficiency of the volumetric blood flow rate in the visceral region for convective transfer to the cooled surface not only of excess heat entering the body from the external environment but also of heat released during metabolic processes. In light of this, it seems expedient to evaluate the efficacy of local abdominal cooling not only against the background of opioid exposure but also during isolated body overheating, with potential revisions to the current “Standard of Emergency Medical Care for Heat and Sun Stroke” if necessary.
However, the data obtained also indicate that local body cooling should not be recommended during acute opioid intoxication under comfortable climatic conditions. It is likely that the decrease in body temperature during local cooling, combined with fentanyl-induced peripheral vasodilation [15] and its direct inhibitory effect on mitochondrial oxygen consumption [16], suppressed the respiratory center and exacerbated hypoxemia, as indicated by cyanosis of the ocular fundus of the animals.
The findings allow us to formulate a hypothesis regarding the mechanisms by which body overheating and cooling of the ventral torso surface against this background affect the toxic effects of fentanyl. Both opioids and heat stress inhibit the propulsive function of the gastrointestinal tract, promoting the overgrowth of thermophilic microflora and its production of toxic substances, including ammonia and lower amines [17]. The accumulation of fluid in the intestinal lumen associated with activation of μ-opioid receptors by fentanyl also stimulates bacterial growth, further facilitating the formation of these substances [18]. The translocation of ammonia and lower amines from the gastrointestinal chyme into the bloodstream is enhanced by μ-opioid receptor agonist-mediated increased intestinal epithelial permeability, which is mediated by Toll-like receptor activation [18]. The subsequent transfer of ammonia and lower amines from the blood into the brain may be facilitated by metabolic acidosis caused by body overheating and acute respiratory failure. The characteristic increase in the pH gradient between blood plasma and cytoplasm intensifies the entry of ammonia and lower amines from the blood into astroglia along the concentration gradient of their neutral gaseous forms [19], which should lead to the accumulation of free amino acids in the brain [20] and cerebral swelling. Due to the inverse relationship between water temperature and the solubility of gases (including ammonia and lower amines), such swelling cannot be alleviated by local head cooling. Conversely, cooling the gastric and intestinal chyme by the magnitude observed in this study — an 11.4 °C drop in body temperature during ice application to the ventral torso surface – should reduce the metabolic activity of thermophilic gastrointestinal microflora by at least 2.2 times, thereby blocking the mechanisms of thanatogenesis mediated by acute intestinal endotoxemia. Testing this hypothesis in further research opens prospects for new approaches to the pathogenetic therapy of both acute opioid intoxication and heat stroke.
CONCLUSIONS
- Body overheating potentiates the lethal and narcotic effects of fentanyl in rats. Under these conditions, cooling the ventral surface of the torso is an effective measure to prevent hyperthermia and reduce lethality, while head cooling is ineffective.
- At room air temperature, local cooling of both the head and the ventral surface of the torso exacerbates fentanyl-induced hypothermia without significantly affecting rat lethality.
- The data obtained should be considered in further research into the influence of climatic factors on the tolerability of narcotic analgesics in larger laboratory animals and humans.
1 Order of the Ministry of Health of the Russian Federation No. 1115n dated 20 Dec. 2012 “Standard of Emergency Medical Care for Heat and Sun Stroke”.
2 Bykov IYu, Rakov AE, Sosyukin AE, eds. Military Field Therapy: A National Guide. Moscow: GEOTAR-Media; 2007.
3 Mashkovsky MD. Medicinal Products. Moscow: Novaya Volna; 2021.
4 Osipov AP, Ibishov DF, Rastorguyeva SL. Physiology of Blood Circulation and Lymph Circulation. Textbook. Perm: IPC ”Prokrost”; 2022.
References
1. Acs A, Schmidt J, Németh Z, Fodor I, Farkas A. Elevated temperature increases the susceptibility of D. magna to environmental mixtures of carbamazepine, tramadol and citalopram. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology. 2025;287:110052. https://doi.org/10.1016/j.cbpc.2024.110052
2. Gordon CJ, Johnstone AFM, Aydin C. Thermal stress and toxicity. Comprehensive Physiology. 2014;4(3):995–1016. https://doi.org/10.1002/cphy.c130046
3. Golovko AI, Ivnitsky JuJu, Rejniuk VL, Schäfer TV. Changes in the illegal synthetic opioids market. Narcology. 2024;23(2):54–9 (In Russ.). EDN: SEMZIT
4. Berezovskaya IV. Prediction of drug safety in preclinical toxicological studies. Toxicological Bulletin. 2010;(5):17–22 (In Russ.). EDN: TQUNAB
5. Shabalov NP, ed. Pediatrics. SPb.: SpecLit; 2003 (In Russ.). EDN: QLFJWL
6. Getsy PM, May WJ, Young AP, Baby SM, Coffee GA, Bates JN, et al. Tropine exacerbates the ventilator depressant actions of fentanyl in freely-moving rats. Frontiers in Pharmacology. 2024;24:1405461. https://doi.org/10.3389/fphar.2024.1405461
7. Wolf ST, Bernard TE, Kenney WL. Heat exposure limits for young unacclimatized males and females at low and high humidity. Journal of Occupational and Environmental Hygiene. 2022;19(7):415–24. https://doi.org/10.1080/15459624.2022.2076859
8. Canfield JR, Sprague JE. Influence of carbon side chain length on the in vivo pharmacokinetic and pharmacodynamics characteristics of illicitly manufactured fentanyls. Drug Testing and Analyses. 2024;16(10):1113–21. https://doi.org/10.1002/dta.3636
9. Attah AT, Negrón-Moreno PN, Amigo-Duran M, Zhang L, Kenngott M, Brecht M, et al. Sensory cues, behavior and furbased drying in the rat wetness response. Scientific Reports. 2024;14(1):24550. https://doi.org/10.1038/s41598-024-74900-9
10. Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti L, et al. Thresholds for thermal damage to normal tissues: an update. International Journal of Hyperthermia. 2011;27(4):320–43. https://doi.org/10.3109/02656736.2010.534527 ORIGINAL ARTICLE | TOXICOLOGY 482 EXTREME MEDICINE | 2025, VOLUME 27, No 4
11. Puszkei A, Malissin I, Cisternino S, Pallet N, Declèves X, Mégarbane B. Massive tramadol ingestion resulting in fatal injury — a pharmacokinetic study with discussion on the involved mechanisms of toxicity. Clinical Toxicology. 2022;60(9):1059–62. https://doi.org/10.1080/15563650.2022.2071286
12. Lee S, Lee SH. Exertional heat stroke with reversible severe cerebral edema. Clinical and Experimental Emergency Medicine. 2021;8(3):242–5. https://doi.org/10.15441/ceem.19.085
13. Alali Z, Eckels SJ. 3D numerical simulations of mixed convective heat transfer and correlation development for a thermal manikin head. Heliyon. 2024;10(9):e30161.
14. Wang J, Jiang C, Kang J, Yu S, Bai G. Head-neck local ventilation mode for long-narrow mine working face. Scientific Reports. 2024;14(1):19663. https://doi.org/10.1038/s41598-024-70739-2
15. Solis E, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology. 2018;43(4):810–9. https://doi.org/10.1038/npp.2017.181
16. Zamparelli M, Eaton S, Quant PA, McEwan A, Spitz L, Pierro A. Analgesic doses of fentanyl impair oxidative metabolism of neonatal hepatocytes. Journal of Pediatric Surgery. 1999;34(2):260–3. https://doi.org/10.1016/s0022-3468(99)90186-0
17. Ivnitsky JuJu, Rejniuk VL, Schäfer ТV, Vakunenkova ОА. Acute intestinal endotoxicosis in disaster medicine. Kremlin Medicine Journal. 2024;1:81–6 (In Russ.). https://doi.org/10.48612/cgma/1e9k-uttr-7h6a
18. Lacy BE, Cangemi DJ. Opioids and the gastrointestinal tract: the role of peripherally active µ-opioid receptor antagonists in modulating intestinal permeability. American Journal of Gastroenterology. 2024;119(10):1970–8. https://doi.org/10.14309/ajg.0000000000002887
19. Ott P, Vilstrup H. Cerebral effects of ammonia in liver disease: current hypotheses. Metabolic Brain Disease. 2014;29(4):901–11. https://doi.org/10.1007/s11011-014-9494-7
20. Zielinska M, Albrecht J, Popek M. Disregulation of astrocytic glutamine transport in acute hyperammonemic brain edema. Frontiers in Neuroscience. 2022;16:875750. https://doi.org/10.3389/fnins.2022.874750
About the Authors
Ju. Ju. IvnitskyRussian Federation
Jury Ju. Ivnitsky
St. Petersburg
O. A. Vakunenkova
Russian Federation
Olga A. Vakunenkova
St. Petersburg
A. I. Golovko
Russian Federation
Alexandr I. Golovko
St. Petersburg
N. V. Lapina
Russian Federation
Nataliya V. Lapina
St. Petersburg
V. L. Rejniuk
Russian Federation
Vladimir L. Rejniuk
St. Petersburg
Supplementary files
Review
For citations:
Ivnitsky J.J., Vakunenkova O.A., Golovko A.I., Lapina N.V., Rejniuk V.L. The effect of general hyperthermia and local cooling on fentanyl tolerance in rats. Extreme Medicine. 2025;27(4):475-482. https://doi.org/10.47183/mes.2025-311

















