Scroll to:
Safety and efficacy of small interfering RNA agents (lumasiran) in therapy for primary hyperoxaluria type 1: A systematic review
https://doi.org/10.47183/mes.2025-344
Abstract
Introduction. Primary hyperoxaluria type 1 (PH1) is an inherited disorder characterized by excessive oxalate production in the liver, leading to hyperoxaluria, kidney stone formation, nephrocalcinosis, and progressive kidney damage. PH1 is caused by mutations in the AGXT gene, whereas types 2 and 3 are associated with mutations in GRHPR and HOGA1, respectively. Lumasiran, an RNA interference (RNAi)-based therapeutic agent, targets the HAO1 gene (hydroxyacid oxidase 1), thus reducing the levels of glycolate oxidase. This action results in decreased hepatic oxalate production.
Objective. Evaluation of the efficacy, safety, and clinical use of lumasiran in adults and children with genetically confirmed primary hyperoxaluria type 1.
Materials and methods. The systematic review was conducted in accordance with the PRISMA 2020 guidelines. A comprehensive literature search was performed across four databases (PubMed, Scopus, Web of Science, and EMBASE). Studies were selected based on their focus on the use of lumasiran in pediatric or adult patients with genetically confirmed primary hyperoxaluria type 1. The quality and risk of bias were assessed using the Joanna Briggs Institute (JBI) critical appraisal tools. The final analysis included 11 studies: two randomized controlled trials, two prospective single-arm studies, one case series (involving five patients), and six individual clinical case reports involving both pediatric and adult populations.
Discussion. Lumasiran treatment was found to lead to a significant reduction in urinary oxalate (UOx) levels (approximately 60–75%) and plasma oxalate (POx) levels (approximately 30–60%). Patients across all age groups, from infants to adults, exhibited markedly stabilized or improved renal function, alongside reduced progression of nephrocalcinosis. Lumasiran demonstrated a favorable safety profile, with the most common adverse events being mild injection-site reactions. No serious treatment-related adverse events requiring discontinuation of therapy were reported.
Conclusions. By suppressing glycolate oxidase expression, lumasiran has consistently demonstrated significant efficacy in reducing oxalate levels. However, there exist differences in therapeutic approaches for adult patients and infants, as well as in treatment effects based on baseline renal function and dosing regimens. Both pediatric and adult populations showed substantial improvement and stabilization of renal function, although infants and patients with advanced chronic kidney disease required dose adjustments. Studies also revealed a greater variability in renal outcomes, particularly regarding the progression of nephrocalcinosis. Although additional large-scale long-term studies are needed, our findings indicate that lumasiran may impede the progression of kidney disease and potentially reduce or delay the need for kidney transplantation in PH1.
Keywords
For citations:
Najafi S., Abasabadi F., Saghafi M.S., Khosravi F., Rahmanian M. Safety and efficacy of small interfering RNA agents (lumasiran) in therapy for primary hyperoxaluria type 1: A systematic review. Extreme Medicine. 2025;27(4):525-535. https://doi.org/10.47183/mes.2025-344
INTRODUCTION
Primary hyperoxaluria (PH) is a rare (orphan) genetically determined autosomal recessive disorder. Its pathogenesis is rooted in impaired hepatic glyoxylate metabolism caused by mutations in the AGXT, GRHPR, and HOGA1 genes, which encode enzymes involved in glyoxylate metabolism [1, 2].
Specifically, primary hyperoxaluria type 1 (PH1) is caused by mutations in the AGXT gene, encoding the enzyme alanine-glyoxylate aminotransferase (AGT). AGT deficiency or dysfunction leads to excessive conversion of glyoxylate to oxalate. This disorder results in:
- overproduction of oxalate in the liver;
- elevated plasma oxalate levels;
- increased urinary oxalate excretion;
- formation of renal calcium-oxalate crystals and radiopaque stones (primarily calcium oxalate monohydrate).
Among the main clinical manifestations are:
- kidney stone formation;
- nephrocalcinosis;
- progressive chronic kidney disease (CKD).
Without intervention, systemic oxalate deposition may occur, potentially leading to end-stage renal disease (ESRD) [3–6].
Due to critical impairments in hepatic enzymatic function and renal excretory capacity, patients with advanced PH1 may require simultaneous or sequential combined liver and kidney transplantation [7]. Therapeutic options for PH1 have conventionally been limited to conservative medical management, including high fluid intake, vitamin B6 (pyridoxine) supplementation, and crystallization inhibitors (e.g., citrate). However, these measures often fail to halt the relentless progression to end-stage renal disease (ESRD) [8].
Isolated kidney transplantation is generally insufficient, as persistent hepatic oxalate production leads to recurrent oxalate nephropathy. Consequently, combined liver-kidney transplantation has become the preferred treatment strategy [3]. Nevertheless, transplantation carries inherent surgical risks, potential graft failure, and immunological complications.
Lumasiran, a small interfering RNA (siRNA)-based therapeutic agent, received approval from the U.S. Food and Drug Administration (FDA) in November 2020 as the first drug indicated for the treatment of primary hyperoxaluria type 1 in adults and children aged six years and older [9]. OxlumoTM (lumasiran) operates via the molecular mechanism of RNA interference (RNAi), inducing degradation of target messenger RNA (mRNA) within the cell cytoplasm and enabling highly specific post-transcriptional gene regulation. This therapeutic approach utilizes small RNA molecules to suppress the expression of specific genes by binding to complementary mRNA sequences and triggering their degradation [10][11].
Lumasiran specifically targets the HAO1 gene, which encodes the hydroxyacid oxidase 1 (HAO1) enzyme in hepatocytes, thereby inhibiting the production of the glycolate oxidase (GO) protein [9][12]. By suppressing GO synthesis, lumasiran reduces hepatic glyoxylate availability and consequently decreases oxalate production, ultimately preventing the accumulation of oxalate crystals in the kidneys and other organs [12].
Clinical trials have demonstrated that lumasiran is highly effective in reducing plasma and urinary oxalate levels, leading to improved renal function in patients with PH1 [13–15]. According to Garrelfs et al., a randomized, double-blind, placebo-controlled clinical trial of Oxlumo™ (lumasiran) showed a significantly greater reduction in 24-h urinary oxalate excretion — 53.5 percentage points more with lumasiran than with placebo over a 6-month treatment period. By month 6, the majority of patients receiving lumasiran achieved urinary oxalate levels within or near the normal range. Furthermore, none of the patients in the lumasiran group developed new kidney stones, whereas kidney stones were detected in 6 out of 12 patients in the placebo group. Additionally, 84% of lumasiran-treated patients exhibited a 24-h urinary oxalate excretion no more than 1.5 times the upper limit of normal by month 6, compared to the placebo group [14].
Lumasiran also demonstrated a favorable safety profile with minimal adverse effects [15]. However, studies indicate that higher doses may be required to ensure efficacy in infants, and treatment may not fully prevent the development of nephrocalcinosis in the long term [16].
While these clinical trial results are promising, further research is needed to fully understand the safety and efficacy of lumasiran for treating hyperoxaluria. These findings may contribute to revised therapeutic protocols and reduce the need for liver transplantation in patients with PH1.
The aim of this study is to evaluate the efficacy, safety, and clinical use of OxlumoTM (lumasiran) in adults and children with genetically confirmed primary hyperoxaluria type 1.
MATERIALS AND METHODS
Study design and search strategy
A systematic review of study results was conducted in accordance with the PRISMA 2020 guidelines [17]. The review protocol was not registered in PROSPERO. A literature search was performed using the PubMed, Scopus, EMBASE, and Web of Science databases. Original studies investigating the use of lumasiran in patients with a genetic or clinical diagnosis of primary hyperoxaluria type 1 were identified.
In the PubMed/Medline database, the search was conducted using Medical Subject Headings (MeSH) terms and keywords: lumasiran, RNAi, primary hyperoxaluria type 1 (PH1), excessive hepatic oxalate, glycolate oxidase inhibition, and small interfering RNA (siRNA). To enhance search efficiency, Boolean operators OR (any of the keywords) and AND (all keywords combined) were used when combining MeSH terms and keywords up to May 2024.
Inclusion and exclusion criteria
The studies included in the systematic review comprised interventional studies (randomized controlled trials, non-comparative studies, and quasi-experimental designs), case series, and clinical case reports containing original data on clinical outcomes of Oxlumo™ (lumasiran) therapy in children and adults with PH1.
The studies excluded from consideration were reviews, animal studies, duplicate full-text publications, conference abstracts without data, and articles with insufficient patient information.
Subsequently, we analyzed sample sizes, patient demographic characteristics, details of PH1 diagnosis, lumasiran dosing and administration regimens, treatment duration, changes in urinary/plasma oxalate levels, renal function assessment data, and all reported adverse events. The studies encompassed patients across a wide age range, from infants (under one year) to elderly adults (50 years and older), with varying degrees of disease severity.
Study selection and data extraction
From the initial 91 articles identified from databases, 53 duplicate publications were excluded. Two independent experts screened the remaining 38 records by title and abstract, excluding 12 irrelevant publications. Two additional articles were included following a search of grey literature via Google Scholar and citation tracking. A total of 26 full-text publications were selected for evaluation, supplemented by 2 additional records from grey literature and citation sources. During screening, 15 articles were excluded due to overlapping data, one publication was a non-systematic review, and one was irrelevant to the topic. The remaining 11 studies met our inclusion criteria.
The systematic review included 11 studies:
- 2 randomized controlled trials,
- 2 non-comparative prospective single-arm studies,
- 1 case series (involving 5 patients),
- 6 individual clinical case reports.
These studies included both pediatric and adult patients, covering age groups from infants (< 1 year) to elderly adults (> 50 years), with varying degrees of PH1 severity.
Quality assessment of included studies
The quality assessment of the included studies was conducted using the approved Joanna Briggs Institute (JBI) critical appraisal checklists, corresponding to the design of each study (randomized controlled trials, case reports, and case series studies). The criteria of each checklist were independently evaluated by two experts, and any discrepancies were resolved through consensus or consultation with a third expert. The majority of the studies demonstrated high methodological quality with minimal bias.
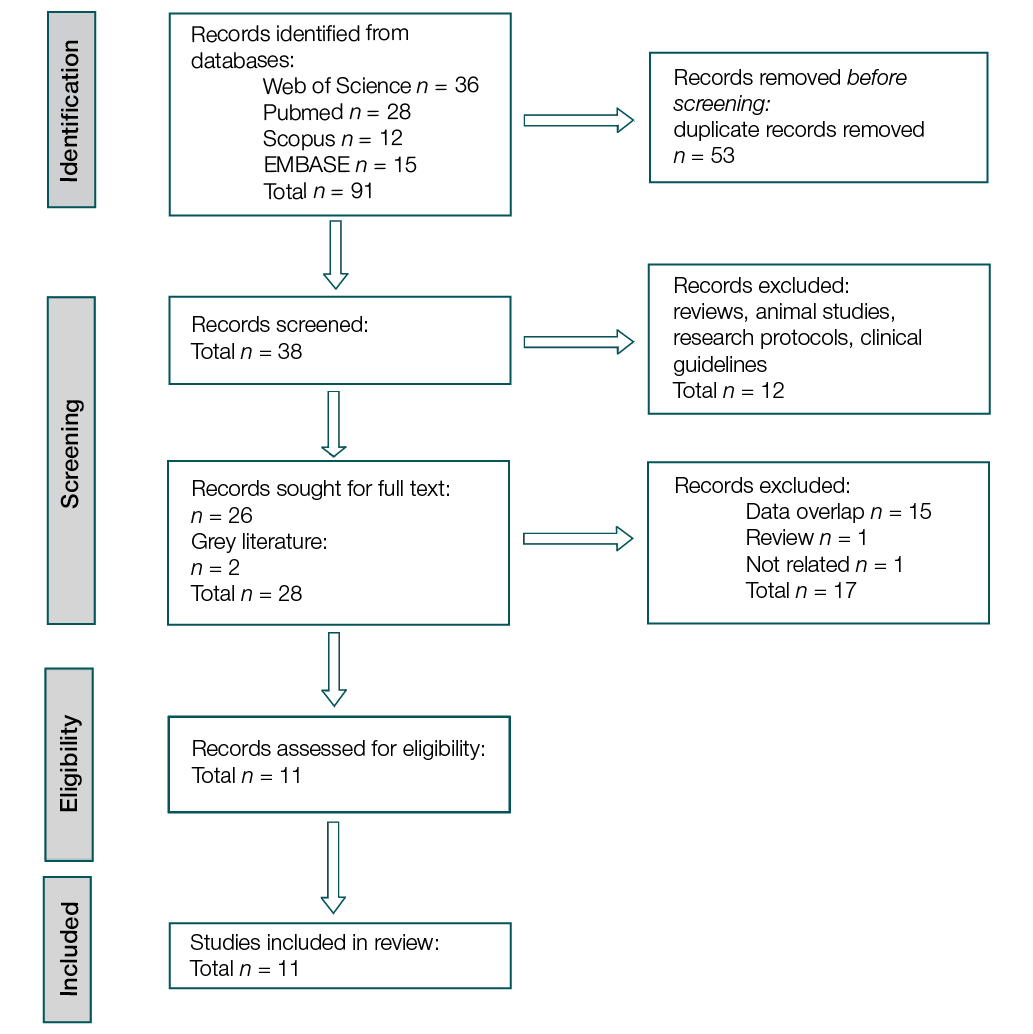
Figure prepared by the authors
Fig. PRISMA flow diagram of the systematic review
RESULTS AND DISCUSSION
The Table summarizes the aggregated study data, patient demographics, Oxlumo™ (lumasiran) dosing regimens, key outcomes/disease progression, and documents a comprehensive analysis of the impact of Oxlumo™ (lumasiran) on the PH1course.
Table. Summary of studies included in the systematic review on the effects of Oxlumo™ (lumasiran) on primary hyperoxaluria type 1 (PH1)
Stu-dy ID | First author, country | Year of publication | Type of Study | Sample size | Application method and dosage regimen for lumasiran | Follow- | Population | Outcomes |
1 | Michael, Israel, France, Germany, the UK, and Netherlands [13] | 2023 | Clinical trial single arm | Total participants: 21 people, 0–59 years | Children weighing <10 kg: 6 mg/kg monthly for three months (loading phase), followed by 3 mg/kg monthly (maintenance phase). Children weighing Children weighing >20 kg: 3 mg/kg monthly for three months (loading phase), followed by 3 mg/kg quarterly (every 3 months) (maintenance phase). All injections were administered subcutaneously. | 6–12 months | Total number of participants: 21 patients. All patients received treatment with lumasiran in two separate cohorts: Cohort A Cohort B | A reduction in POx levels of 33.3% and 42.4% was observed, alongside an acceptable safety profile for patients |
2 | Garrelfs, the Netherlands [14] | 2021 | RCT | Total Participants: 39 individuals, aged 6–47 years | 3mg/kg monthly for 3 months. Followed by maintenance doses given once every 3 months, beginning 1 month after the last loading dose, followed for 6 months. All injections were performed subcutaneously | 6 months | Total participants: Lumasiran group: n = 26 (31% female; 69% male). Placebo group: n = 13 (38% female; 62% male) | 64% reduction in 24-hour UOx excretion (84% below 1.5 times the upper limit of normal) Reduction in POx levels eGFR remained stable Decrease in UOx/Cr ratio |
3 | Méaux, France [16] | 2022 | Case report | Total number of participants: 3 | Dosing regimen: 6 mg/kg monthly for 3 months (loading phase), followed by a reduction to 3 mg/kg monthly (maintenance phase) for children weighing less than 10 kg. All injections were administered subcutaneously. | 10 months | Infants before 2 years of age | Reduction in POx levels. Decrease in UOx/Cr ratio. Renal function remained normal |
4 | Frishberg, Israel, France, Germany, the UK, and the Netherlands [18] | 2021 | RCT | Total number of participants: 52 individuals, aged 6–64 years | Dosing and Administration Regimens: • 1 mg/kg once monthly; • 3 mg/kg once monthly; • 3 mg/kg every 3 months. Observation period: At least 12 weeks. All injections were administered subcutaneously | 85 days, 197 days | Total number of participants: Healthy volunteers: n = 32 • Lumasiran group: n = 24 (46% female; 54% male) • Placebo group: n = 8 (63% female; 37% male) Patients: • Lumasiran group: n = 17 (71% female; 29% male) • Placebo group: n = 3 (33% female; 67% male) | A 75% reduction in 24-hour UOx excretion (≤ 1.5 times the upper limit of normal). A decrease in POx concentration |
5 | Sas, Israel, France, Germany, the UK, and the Netherlands [19] | 2022 | Clinical trial single arm | Total number of participants: 18, aged 0 months to 6 years | Dosing regimen for pediatric patients: • Children weighing <10 kg: 6 mg/kg monthly for three months (loading phase), followed by 3 mg/kg monthly (maintenance phase). • Children weighing 10 kg to <20 kg: 6 mg/kg monthly for three months (loading phase), followed by 6 mg/kg quarterly (every 3 months) (maintenance phase). • Children weighing >20 kg: 3 mg/kg monthly for three months (loading phase), followed by 3 mg/kg quarterly (every 3 months) (maintenance phase). All injections were administered subcutaneously | 6 months | Total number of participants: 18 patients. All patients received treatment with lumasiran. Stratified by weight group: • <10 kg: n = 3 (33% female); • 10 to <20 kg: n = 12 (75% female); • ≥20 kg: n = 3 (0% female). All treated patients (pooled): n = 18 (56% female) | A 72% reduction in UOx/Cr and a decrease in POx levels in children under 6 years of age (50% lower than 1.5 times the upper limit of normal) |
6 | Aldabek, the USA [20] | 2022 | Case report | Total participants: 2 | Dosing regimen: 6 mg/kg | 8 months | Two male twins, | Significant improvement in symptoms |
Lombardi, France [21] | 2023 | Case report | Total participants: 1 | Dosing regimen: 3 mg/kg | 14 months | A male patient 51 years old | Decrease of SOx and UOx concentration, as well as a decrease in oxalate crystal deposition in the kidneys | |
Sellier –Leclerc, France [22] | 2023 | Case series | Total number of participants: 5, aged 3–45 years | Lumasiran was administered via subcutaneous injections monthly for 3 months (loading phase), followed by maintenance dosing every 3 months. Data on the exact dosage are unavailable | 13 months | Total number of participants: 5 patients. All patients received treatment with lumasiran | Reduction of POx level | |
7 | Chiodini, Belgium [23] | 2022 | Case report | Total participants: 1 | Dosing Regimen: 3 mg/kg | 18 months | Patient boy, 13 years old | Reduction of POx and UOx levels to within normal range. 70% reduction in UOx/Cr ratio. eGFR remained stable (60 mL/min/1.73 m2) |
10 | Joher, France [24] | 2022 | Case report | Total participants: 1 | Lumasiran therapy before KTx | Duration not detailed | 39 years old women | Normalization of SOx concentration before KTx |
11 | Poyah, Canada [25] | 2021 | Case report | Total participants: 1 (adult, ESKD, cutaneous manifestations) | Not specified | Duration not detailed | A 40-year-old female with primary hyperoxaluria (PH), suffering from end-stage renal disease (ESRD) with cutaneous manifestations | POx level decreased by 36%, but renal function did not recover; progression of extrarenal involvement with swan-neck deformity and pulmonary hypertension was observed |
Table prepared by the authors using data from Ref. [13][14][16][18–25]
Note: RCT — randomized controlled trial; NRCT — non-randomized controlled trial; POx — plasma oxalates; UOx / Cr — the ratio of oxalates in urine and creatinine level; UOx — urinary oxalates; SOx — serum oxalates; KTx — renal transplantation; eGFR — glomerular filtration rate.
Frishberg et al. [18] reported a significant reduction in mean maximum 24-h Urinary oxalate (UOx) excretion levels by 75%, or 43–92% from the baseline value of 1.69 mmol/24 h/1.73 m2. Notably, all study participants achieved UOx levels ≤ 1.5 times the upper limit of normal (ULN). This confirms that the core mechanism of lumasiran lies in its ability to degrade glycolate oxidase mRNA. Collectively, these results provide a comprehensive understanding of the efficacy of lumasiran in alleviating the course of PH1.
Garrelfs et al. [14] evaluated the effect of lumasiran therapy on changes in 24-h urinary oxalate excretion and plasma oxalate (POx) levels in PH1 patients. The data revealed that 84% of lumasiran-treated patients achieved 24-h UOx levels ≤ 1.5 times the ULN. Furthermore, lumasiran treatment demonstrated a significant reduction in POx levels, providing compelling evidence of its established mechanism of action.
Michael et al. [13] observed that lumasiran administration led to a marked decrease in POx levels while maintaining a favorable safety profile in individuals with progressive kidney disease and PH1.
Sas et al. [19] conducted a study investigating the efficacy of lumasiran as a therapeutic agent for treating PH1 in pediatric patients. The study utilized a regimen of 4 or 6 loading doses of the drug, adjusted according to the patient weight. The results demonstrated a 72.0% reduction in the oxalate-to-creatinine excretion ratio (UOx:Cr). Furthermore, half of the patients achieved UOx:Cr values within half of the upper limit of normal (ULN). The reduction in POx reached 31.7%. To evaluate the impact of lumasiran therapy on PH1, six clinical studies were included in the analysis.
Méaux et al. [16] in their study observed three infants diagnosed with PH1. The patients received lumasiran therapy, with dosage and frequency adjusted based on the child’s body weight. For the first 3 months, a dose of 6 mg/kg per month was prescribed; for infants weighing less than 10 kg, this regimen was adjusted to 3 mg/kg per month. As explained by Méaux et al., this method underscores the importance of weight-based factors in determining the appropriate lumasiran dose for infants with PH1. Patient 1 was diagnosed with PH1 prenatally because his older sister was diagnosed with stage 5 chronic kidney disease (CKD) at 4 months of age. After 10 months of observation, renal hyperechogenicity in the patient began to decrease, with preserved kidney function. Patient 2, diagnosed with PH1, was hospitalized due to acute renal failure and dehydration at 2.5 months of age. Serum creatinine levels were 243 μmol/L, blood urea nitrogen 19 mmol/L, with an estimated glomerular filtration rate (eGFR) of 8 mL/min/1.73 m2, and UOx:Cr ratio (806 μmol/mmol) and POx (184 μmol/L), which were significantly elevated. After nine injections, the UOx:Cr ratio decreased by more than 60% — to 310 μmol/mmol, which was nearly normal. During the 10-month observation period, a sharp decline in serum creatinine levels was noted, eventually stabilizing at approximately 120 μmol/L (eGFR 20 mL/min/1.73 m2). However, grade III nephrocalcinosis persisted. Due to the presence of grade III nephrocalcinosis at 3.5 months of age, patient 3 was enrolled in the study with a diagnosis of PH1. After one week, the UOx:Cr ratio increased to 2167 μmol/mmol from an elevated baseline of 1651 μmol/mmol, according to biochemical analysis. POx level was 36 μmol/L, accompanied by an elevated plasma glycolate level, but normal kidney function (creatinine 30 μmol/L, eGFR 77 mL/min/1.73 m2). After the initial administration, a rapid decrease in the UOx:Cr ratio to 1640 μmol/mmol was observed. Kidney function remained stable throughout the observation period. After the fifth injection, nephrocalcinosis decreased from grade III to grade II. The results indicate that lumasiran is effective in infants, exhibiting no negative side effects. However, despite the good tolerability of lumasiran, it is not possible to completely avoid the occurrence or progression of nephrocalcinosis, especially in its severe forms, even when therapy is initiated in the early neonatal period or combined with standard approaches to treating PH1 [22].
In a study conducted by Aldabek et al. [20], the focus was on two male twin infants diagnosed with PH1 who exhibited symptoms of nephrolithiasis and nephrocalcinosis. These patients received lumasiran treatment starting at 12 months of age, with an initial dose of 6 mg/kg once monthly for the first three months, followed by an adjustment to 3 mg/kg monthly. Notably, the twin boys showed significant symptomatic improvement. Based on these positive outcomes, Aldabek et al. concluded that lumasiran is a successful treatment for pediatric PH1.
Chiodini et al. [23] observed an adolescent patient with PH1 who received lumasiran at a dose of 3 mg/kg over 18 months. The patient exhibited a rapid and sustained reduction in the UOx:Cr ratio, averaging 70% after lumasiran administration. Throughout the 18-month observation period, UOx levels remained low, nearly approaching the normal range. Additionally, a rapid decline in POx levels was observed, with an average reduction of approximately 60% following lumasiran treatment. The estimated glomerular filtration rate (eGFR) showed no significant changes over the entire treatment period, ranging from 60 mL/min/1.73 m2 at baseline to 62 mL/min/1.73 m2 at 18 months.
Lombardi et al. [21] studied the efficacy of lumasiran therapy in a 51-year-old patient with PH1 who experienced recurrent oxalate nephropathy after an isolated kidney transplant. The drug therapy involved subcutaneous administration of lumasiran at a dose of 3 mg/kg. A total of three-monthly injections were administered initially, followed by injections every three months. After initiating lumasiran, a reduction in serum oxalate (SOx) concentration, urinary oxalate, and renal oxalate crystal deposition was observed.
Another study conducted by Sellier-Leclerc et al. [22] included five patients with genetically confirmed PH1 who had undergone isolated kidney transplantation. The patients, with a mean age of 26 years (range 3–45 years), received lumasiran therapy for a median duration of 13 months (range 5–17 months). The results showed a consistent and significant reduction in POx levels in all patients after initiating lumasiran: from 110 (20–150) μmol/L to 53 (10–72) μmol/L at the time of kidney transplantation (KTx), and further to 7 (5–26) μmol/L at three months post-treatment (p < 0.05). Thus, in cases where the POx level ranges 80–90 μmol/L, the findings suggest that isolated KTx combined with lumasiran therapy may be a safe treatment option for PH1 patients with renal failure.
Joher et al. [24] reported a 39-year-old female with PH1 and a history of kidney transplantation (KTx) who had previously received lumasiran therapy. The results showed that SOx concentration normalized even before the KTx surgery. Lumasiran therapy led to favorable outcomes, including reductions in SOx, POx, 24-h UOx, and the UOx:Cr ratio. This was achieved through degradation of mRNA encoding glycolate oxidase, the enzyme regulating AGT, thereby reducing oxalate production.
In a study by Poyah [25], a clinical case of primary hyperoxaluria type 1 was described in a 40-year-old female with a history of recurrent nephrolithiasis. Lumasiran therapy was initiated 11 months after starting hemodialysis and pyridoxine treatment. After 14 months of high-intensity hemodialysis and three months of lumasiran, no signs of renal recovery were observed, and extrarenal complications worsened, including progressive swan-neck deformities, reduced systolic heart function, and pulmonary hypertension. The patient was placed on the waiting list for combined liver–kidney transplantation.
The primary side effect associated with the use of lumasiran was mild and transient injection site reactions. Typical signs and manifestations included redness, skin discoloration, and hematoma at the injection site [18][23][25]. During studies, some patients experienced minor adverse effects, including fever, vomiting, rhinitis, abdominal pain, diarrhea, anemia, headache, or accidental overdose [13]. It is suggested that lumasiran does not have any clinically significant impact on laboratory results (including blood tests and liver function), ECG, or other vital signs [23]. This confirms that lumasiran therapy is a safe and effective treatment for infants, young children, and adults.
In our work, we studied the efficacy, safety, and clinical outcomes of lumasiran in the treatment of PH1. Our analysis of 11 studies, including randomized controlled trials, clinical case reports, and case series established that lumasiran, an RNA interference-based drug, significantly reduces oxalate levels in both plasma and urine, stabilizes or modestly improves renal function, and reduces nephrocalcinosis in patients of various ages, including adults and children. It was found that most patients achieved normal or near-normal oxalate levels while using the drug. The drug was generally well tolerated, with the most commonly reported side effect being mild injection site reactions. Thus, lumasiran represents a promising breakthrough in the treatment of PH1. Long-term follow-up data (>3 years) remain limited, particularly for infants, and further monitoring is essential to assess sustained efficacy and renal outcomes.
Across all the reviewed studies, lumasiran consistently demonstrated significant efficacy in reducing oxalate levels. However, variations were observed in patient age, baseline renal function, and dosing regimens. Both children and adults showed substantial improvement and normalization of renal function, although infants and patients with progressive chronic kidney disease required dose adjustments. The studies also revealed a greater variability in renal outcomes, particularly regarding the progression of nephrocalcinosis.
Lumasiran acts by suppressing the HAO1 gene, which encodes glycolate oxidase—an enzyme involved in oxalate production [9]. Consequently, by inhibiting glycolate oxidase, the substrate required for oxalate production is reduced, while the levels of calcium glycolate, a less harmful metabolite, increase [17]. This reduction in oxalate synthesis leads to decreased oxalate levels in both blood and urine [25]. Numerous clinical trials and case reports have confirmed these effects and their clinical implications, such as improved renal function in both pediatric and adult patients with PH1 [13][18][25]. Pharmacokinetic studies indicate that lumasiran is rapidly absorbed and eliminated, supporting its favorable safety profile [9].
In the Phase III open-label single-arm study (ILLUMINATE-B) conducted in 2021, 18 children under six years of age with PH1 received lumasiran treatment for six months and demonstrated a rapid reduction in oxalate concentrations, ultimately reaching the upper limit of normal [18].
According to the ILLUMINATE-A study, kidney stone formation decreased after 6–12 months of lumasiran treatment in PH1 patients over six years of age [18][11]. Urinary oxalate excretion also normalized [14].
Furthermore, the efficacy of lumasiran was evaluated in patients of various age groups and those with progressive CKD over 12 months in the ILLUMINATE-C study [13][17]. As a result, POx concentrations were significantly reduced. This may delay the need for dialysis and transplantation in CKD patients and improve the prognosis for those who have already undergone kidney transplantation [28]. Regarding renal function, after several months of lumasiran treatment, eGFR remained stable or even improved [18][28].
In summary, lumasiran may impede the progression to end-stage renal failure by improving kidney function [20][23]. However, the optimal timing for initiating lumasiran remains unclear. While early treatment may help prevent the accumulation of oxalate crystals in the kidneys and other organs and slow the progression of nephrocalcinosis, it does not completely prevent these manifestations in some patients [23]. Therefore, further research is needed to clarify the goals of comprehensive therapy.
Another significant advantage of lumasiran consists in its favorable tolerability profile. The most frequently reported adverse events were transient, mild injection site reactions [14][18]. Some patients experienced at least one manageable minor side effect, including fever, vomiting, rhinitis, abdominal pain, upper respiratory tract infection, diarrhea, anemia, ear infection, headache, or accidental overdose, all of which resolved rapidly during the study [13]. It appears promising that lumasiran has no clinically relevant impact on laboratory results (including hematological and liver function tests), electrocardiograms, or vital signs [23]. The studies [23] reported neither serious safety concerns—such as treatment discontinuation or drug-related deaths, nor worsening of severe symptoms [15]. Symptoms such as fatigue, nausea, reduced appetite, bone pain, decreased mobility, shortness of breath, renal colic, and others either improved or remained unchanged during lumasiran treatment [15].
While studies on the use of lumasiran for treating PH1 are promising, certain limitations and areas requiring further investigation remain. A notable drawback of many studies reviewed in our work is their sample size, frequently involving only one or several patients. In order to gain deeper insights into the safety and efficacy of this drug, larger randomized controlled trials are necessary. Additional research is also needed across diverse age groups, such as young children and elderly patients, as well as specific patient subgroups, including those with progressive kidney disease.
Furthermore, there is a lack of long-term data on the effects of lumasiran beyond one year, which is critical for understanding the full benefits and potential risks of this therapy. The optimal dosage and treatment schedule have not yet been definitively established, particularly for pediatric patients. Although early initiation of treatment may prevent or reduce the development of nephrocalcinosis in some children, it does not fully eliminate the condition.
While lumasiran therapy appears to reduce oxalate deposition in the kidneys, further studies are needed to determine its impact on extrarenal oxalate deposition. Additionally, most existing studies were conducted in specialized centers using advanced PH1 treatment protocols, which may limit the generalizability of their findings.
Finally, combination therapy involving lumasiran and adjunctive medications may offer additional benefits; however, its potential remains to be elucidated. More clinical trials are required to enable meaningful meta-analyses. We therefore recommend conducting additional systematic reviews alongside meta-analyses to comprehensively evaluate the evidence.
In summary, while lumasiran demonstrates significant potential as a promising treatment for PH1, future large-scale studies or registry-based trials will be essential to confirm its efficacy, safety, and broader applicability. Key priorities include determining optimal dosing for neonates and patients with advanced-stage CKD, and evaluating whether combination therapies can reliably prevent the need for liver and kidney transplantation.
CONCLUSION
Our findings underscore the high efficacy and favorable safety profile of lumasiran, a breakthrough RNAi-based medication that reduces plasma and urinary oxalate levels, thereby preventing kidney damage in patients with primary hyperoxaluria type 1 (PH1), across both pediatric and adult populations. Lumasiran is primarily associated with mild, transient injection-site reactions and is emerging as a first-line therapy for PH1.
While lumasiran demonstrates robust oxalate-lowering effects, variations exist in its therapeutic application:
- age-based considerations: dosing and response differ between adults and infants, with weight-adjusted regimens critical for young children;
- renal function dependence: efficacy and dosing must be tailored to baseline kidney function, particularly in patients with advanced chronic kidney disease (CKD);
- heterogeneous renal outcomes: improvements in renal function are consistently observed, but variability persists in such metrics as nephrocalcinosis progression, especially in severe cases.
The results indicate that lumasiran can impede the progression of kidney disease and potentially reduce or delay the need for transplantation in PH1 patients. However, large-scale long-term studies are still needed to confirm these findings. Future research should focus on:
1) determining the optimal timing for therapy initiation, particularly during infancy;
2) evaluating the potential additive effects of combination therapies (e.g., with pyridoxine or conservative measures);
3) validating its durable benefits and safety over extended follow-up periods.
Further studies will provide evidence-based data to support broader clinical adoption of lumasiran, thereby enhancing understanding of treatment strategies, long-term prognoses, and outcomes for PH1 patients.
References
1. Huang Y, Zhu W, Zhou J, Huang Q, Zeng G. Navigating the Evolving Landscape of Primary Hyperoxaluria: Traditional Management Defied by the Rise of Novel Molecular Drugs. Biomolecules. 2024;14(5):511. https://doi.org/10.3390/biom14050511
2. Cellini B. A molecular journey on the pathogenesis of primary hyperoxaluria. Current Opinion in Nephrology and Hypertension. 2024;33(4):398–404. https://doi.org/10.1097/MNH.0000000000000987
3. Demoulin N, Aydin S, Gillion V, Morelle J, Jadoul M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation. 2022;79(5):717–27. https://doi.org/10.1053/j.ajkd.2021.07.018
4. Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, et al. Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association — European Renal Association. 2012;27(5):1729–36. https://doi.org/10.1093/ndt/gfs078
5. Amoroso A, Pirulli D, Florian F, Puzzer D, Boniotto M, Crovella S, et al. AGXT gene mutations and their influence on clinical heterogeneity of type 1 primary hyperoxaluria. JASN. 2001;12(10):2072–9. https://doi.org/10.1681/ASN.V12102072
6. Hoppe B. An update on primary hyperoxaluria. Nature reviews. Nephrology. 2012;8(8):467–75. https://doi.org/10.1038/nrneph.2012.113
7. Devresse A, Cochat P, Godefroid N, Kanaan N. Transplantation for Primary Hyperoxaluria Type 1: Designing New Strategies in the Era of Promising Therapeutic Perspectives. Kidney International Reports. 2020;5(12):2136–45. https://doi.org/10.1016/j.ekir.2020.09.022
8. Gupta A, Somers MJG, Baum MA. Treatment of primary hyperoxaluria type 1. Clinical Kidney Journal. 2022;15(Suppl. 1):i9–13. https://doi.org/10.1093/ckj/sfab232
9. Scott LJ, Keam SJ. Lumasiran: First Approval. Drugs. 2021;81(2):277–82. https://doi.org/10.1007/s40265-020-01463-0
10. Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, et al. Therapeutic siRNA: state of the art. Signal Transduction and Targeted Therapy. 2020;5(1):101. https://doi.org/10.1038/s41392-020-0207-x
11. D’Ambrosio V, Ferraro PM. Lumasiran in the Management of Patients with Primary Hyperoxaluria Type 1: From Bench to Bedside. International Journal of Nephrology and Renovascular Disease. 2022;15:197–206. https://doi.org:10.2147/IJNRD.S293682
12. Hulton SA. Lumasiran: expanding the treatment options for patients with primary hyperoxaluria type 1. Expert Opinion on Orphan Drugs. 2021;9(7–10):189–98. https://doi.org/10.1080/21678707.2021.2003779
13. Michael M, Groothoff JW, Shasha-Lavsky H, Lieske JC, Frishberg Y, Simkova E, et al. Lumasiran for Advanced Primary Hyperoxaluria Type 1: Phase 3 ILLUMINATE-C Trial. American Journal of Kidney Disease. 2023;81(2):145–55.e1. https://doi.org:10.1053/j.ajkd.2022.05.012
14. Garrelfs SF, Frishberg Y, Hulton SA, Koren MJ, O’Riordan WD, Cochat P, et al. ILLUMINATE-A Collaborators. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. The New England Journal of Medicine. 2021;384(13):1216–26. https://doi.org/10.1056/NEJMoa2021712
15. Hulton SA, Groothoff JW, Frishberg Y, Koren MJ, Overcash JS, Sellier-Leclerc AL, et al. Randomized Clinical Trial on the Long-Term Efficacy and Safety of Lumasiran in Patients with Primary Hyperoxaluria Type 1. Kidney International Reports. 2022;7(3):494–506. https://doi.org/10.1016/j.ekir.2021.12.001
16. Méaux MN, Sellier-Leclerc AL, Acquaviva-Bourdain C, Harambat J, Allard L, Bacchetta J. The effect of lumasiran therapy for primary hyperoxaluria type 1 in small infants. Pediatric Nephrology. 2022;37(4):907–11. https://doi.org/10.1007/s00467-021-05393-1
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
18. Frishberg Y, Deschenes G, Groothoff JW, Hulton SA, Magen D, Harambat J, et al. Phase 1/2 Study of Lumasiran for Treatment of Primary Hyperoxaluria Type 1: A PlaceboControlled Randomized Clinical Trial. Clinical Journal of the American Society of Nephrology. 2021;16(7):1025–36. https://doi.org/10.2215/CJN.14730920
19. Sas DJ, Magen D, Hayes W, Shasha-Lavsky H, Michael M, Schulte I, et al. Phase 3 trial of lumasiran for primary hyperoxaluria type 1: A new RNAi therapeutic in infants and young children. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2022;24(3):654–62. https://doi.org/10.1016/j.gim.2021.10.024
20. Aldabek K, Grossman OK, Al-Omar O, Fox JA, Moritz ML. Infantile Primary Hyperoxaluria Type 1 Treated With Lumasiran in Twin Males. Cureus. 2022;14(1):e21673. https://doi.org/10.7759/cureus.21673
21. Lombardi Y, Isnard P, Chavarot N, Chauvet S, Martinez F, Thervet É, et al. Stiripentol and Lumasiran as a Rescue Therapy for Oxalate Nephropathy Recurrence After Kidney Transplantation in an Adult Patient With Primary Hyperoxaluria Type 1. American Journal of Kidney Diseases. 2023;82(1):113–6.10. https://doi.org/10.1053/j.ajkd.2022.12.005
22. Sellier-Leclerc AL, Metry E, Clave S, Perrin P, AcquavivaBourdain C, Levi C, et al. Isolated kidney transplantation under lumasiran therapy in primary hyperoxaluria type 1: a report of five cases. Nephrology Dialysis Transplantation. 2023;38(2):517–21. https://doi.org/10.1093/ndt/gfac295
23. Chiodini B, Tram N, Adams B, Hennaut E, Lolin K, Ismaili K. Case Report: Sustained Efficacy of Lumasiran at 18 Months in Primary Hyperoxaluria Type 1. Frontiers in Pediatrics. 2021;9:791616. https://doi.org/10.3389/fped.2021.791616
24. Joher N, Moktefi A, Grimbert P, Pagot E, Jouan N, El Karoui K, et al. Early post-transplant recurrence of oxalate nephropathy in a patient with primary hyperoxaluria type 1, despite pretransplant lumasiran therapy. Kidney International. 2022;101(1):185–6. https://doi.org/10.1016/j.kint.2021.10.022
25. Poyah P, Bergman J, Geldenhuys L, Wright G, Walsh NM, Hull P, et al. Primary Hyperoxaluria Type 1 (PH1) Presenting With End-Stage Kidney Disease and Cutaneous Manifestations in Adulthood: A Case Report. Canadian Journal of Kidney Health Diseases. 2021;8. https://doi.org/10.1177/20543581211058931
26. Garrelfs S, Frishberg Y, Hulton S, Koren M, O’Riordan W, Cochat P, et al. LB002 ILLUMINATE-A, a phase 3 study of lumasiran, an investigational RNAI therapeutic, in children and adults with primary hyperoxaluria type 1 (PH1). Nephrology Dialysis Transplantation. 2020;35(Supplement 3):gfaa146. LB002. https://doi.org/10.1093/ndt/gfaa146.LB002
About the Authors
S. NajafiIslamic Republic of Iran
Sara Najafi
Mashhad
F. Abasabadi
Islamic Republic of Iran
Fatemeh Abasabadi
Tehran
M. S. Saghafi
Islamic Republic of Iran
Mohammad Sadra Saghafi
Qom
F. Khosravi
Islamic Republic of Iran
Farbod Khosravi
Tehran
M. Rahmanian
Islamic Republic of Iran
Mohammad Rahmanian
Tehran
Supplementary files
Review
For citations:
Najafi S., Abasabadi F., Saghafi M.S., Khosravi F., Rahmanian M. Safety and efficacy of small interfering RNA agents (lumasiran) in therapy for primary hyperoxaluria type 1: A systematic review. Extreme Medicine. 2025;27(4):525-535. https://doi.org/10.47183/mes.2025-344

















