Scroll to:
Prospects for the use of alpha-2-macroglobulin as a radioprotective agent
https://doi.org/10.47183/mes.2025-316
Abstract
Introduction. The diversity of clinical manifestations of radiation sickness creates significant difficulties in the development of a versatile means for the prevention and treatment of radiation injuries.
Objective. Assessment of the prospects for using alpha-2-macroglobulin (α2M) as a radioprotective agent.
Discussion. The existing agents were established to be incapable of simultaneous implementation of multiple mechanisms of radioprotective action, rendering the development of complex formulations the primary research direction. However, the toxicity, side effects, and multidirectional nature of many radioprotectors hinders their combined application. Along with inhibiting proteinases, alpha-2-macroglobulin (α2M) is involved in lipid metabolism and regulation of the antioxidant system. It influences enzyme activity, binds and transports numerous cytokines, affects the functions of immunocompetent cells, and controls the development of the inflammatory response and tissue remodeling processes. A number of published studies confirm α2M to be a promising radioprotector and a key component of innate radioprotection.
Conclusions. Preparations based on human blood polyfunctional proteins can serve as a basis for the development of means for preventing and treating radiation injuries. The α2M administration into the body reduces lethality, protects DNA from damage, lowers the oxidative stress level, mitigates the severity of leukopenia and thrombocytopenia, and reduces the number of necrosis foci. Further research into the radioprotective properties of this protein and the optimization of methods for its isolation from blood for industrial-scale production are required.
Keywords
For citations:
Zorina V.N., Evdokimova E.A. Prospects for the use of alpha-2-macroglobulin as a radioprotective agent. Extreme Medicine. 2025;27(4):516-524. https://doi.org/10.47183/mes.2025-316
INTRODUCTION
The current stage of technological development is associated with aggravation of radiation hazards to the level of public discussions around the possibility of using tactical nuclear weapons. At the same time, the delayed effects of radiation sickness are generally ignored, ranging from acute leukemia, which developed en masse 9–10 days after the bombings of Hiroshima and Nagasaki [1], to teratogenic effects observed in civilians living for many years in areas adjacent to territories contaminated with depleted uranium (Iraq, Serbia, Libya, Somalia, Haiti, etc.) [2]. It should be noted that the existing technologies aimed at ecosystem restoration and human safety, including biorevitalization and biomineralization methods, demonstrate insufficient efficiency [3]. Undoubtedly, the tragedy of Hiroshima and Nagasaki was a harsh lesson to learn for contemporaries, intensifying experimental studies of radiation sickness in laboratory animals, as well as the development of personal protective equipment and medical means of radiological protection.
Today, the biological effects of ionizing radiation are viewed as a combination of molecular, biochemical, morphological, physiological, and genetic changes. Numerous deterministic and stochastic (dose-independent) effects have been described and studied [4]. Overall, radiation pathology is characterized by a diversity of clinical forms, hindering the creation of a unified classification of radiation injuries. In addition to the duration of exposure and dose, the routes of radionuclide intake into the body, distribution features, tropism to organs and tissues, and the ability to adapt and regenerate at the individual level are of great importance for pathogenesis. With an increase in the absorbed dose, the bone marrow and intestines are sequentially affected, vascular-toxemic, cardiovascular, and cerebral forms of acute radiation sickness develop, with a corresponding reduction in the time to lethal outcome (a dose of up to 10 Gray is considered treatable) [4]. The diversity of clinical manifestations of radiation sickness creates significant barriers to the development of versatile prevention and treatment means.
The aim of this work is to assess the prospects for using alpha-2-macroglobulin as a radioprotective agent.
MATERIALS AND METHODS
The literature search was carried out in electronic bibliographic databases in the Russian (E-library, CyberLeninka) and English (PubMed) languages and patent sources (FIPS, EspaceNet). Search queries included the following keywords: alpha-2-macroglobulin, radioprotector, radioprotection, anti-radiation. The search depth for the combination of keywords “alpha-2-macroglobulin” and others was not specified, and the search depth for the remaining keywords was 10 years.
RESULTS AND DISCUSSION
Existing and novel radioprotective agents
Medicinal products used for the prevention and treatment of radiation injuries lack a unified classification; however, they can be conventionally divided into radioprotectors, agents for long-term enhancement of body resistance, and means for injury prevention. Based on the scenarios of exposure, they are distinguished as radioprotectors (providing short-term effects), radiomitigators (long-term exposure, stimulation of repair), radiomodifiers (non-specific enhancement of body resistance), agents preventing incorporation and promoting the elimination of radionuclides from the body, and means for suppressing undesirable body reactions to irradiation. Medical means are also conventionally subdivided into preventive (radioprotectors, stimulators of body radioresistance), therapeutic-prophylactic (radiomitigators, agents for alleviating the primary body reaction and means for preventing effects from incorporated nucleotides), and therapeutic agents (treatment of acute bone marrow syndrome, skin and mucous membrane injuries). Additionally, classifications based on the biological activity of the agents and other criteria exist [4][5].
It is important to note that none of the hypotheses regarding the radioprotective action of pharmacological compounds allow for a unified theoretical generalization of the mechanism of their action, as none of the recommended or developed agents enable the simultaneous implementation of multiple mechanisms of radioprotective action [6]. In Russia, indralin (B-190) [4][6], a biogenic amine, is recommended as a radioprotector. The high protective efficacy of indralin is primarily associated with its vasoconstrictor activity, leading to regional impairment of blood supply, including in radio-sensitive tissues. It has been suggested that B-190 provokes tissue hypoxia by activating tissue respiration through α1-adrenergic receptors. A radiomitigating effect has also been assumed; thus, indralin indirectly releases serotonin from bone marrow tissues, and serotonin, in turn, stimulates the proliferation of hematopoietic stem cells. However, the radiomitigating properties of indralin, while contributing to increased radioresistance, are still not comparable to its radioprotective effect [6].
Along with B-190, naphthyzin, a common alpha-adrenomimetic with vasoconstrictive action, is also used as a radioprotector. Recommended as a means for preventing and alleviating the primary radiation reaction, ondansetron hydrochloride dihydrate (Latran®, granisetron) is essentially an antiemetic drug [5][6]. Enterosorbents and other means for preventing injuries from incorporated radionuclides, including potassium iodide, potassium-iron hexacyanoferrate (Ferrocyn®), calcium-trisodium salt of diethylenetriaminepentaacetic acid (Pentacin), and 2,3-dimercaptopropanesulfonate (Unithiol) [5][6], protect only specific organs and have application limitations, with many enterosorbents developed in the USSR long being discontinued.
The attempts to develop and test novel means of radiation protection are ongoing. Some previously developed anti-radiation agents (cystamine, Mexamine®) have been surpassed in terms of tolerability and protective properties by modern radioprotectors and are seeking new medical applications [6]. Efforts are being made to reduce the side effects of amifostine (WR-2721 is the primary radioprotector in the USA and Western Europe) by modifying the active component. Thus, a new polycysteine peptide with three thiol groups has been synthesized to reduce toxicity, demonstrating efficacy comparable to that of amifostine and a better safety profile [7].
Among original innovations, the proposal to use molecular hydrogen for radioprotection (as an antioxidant, anti-inflammatory, anti-apoptotic agent, and a factor influencing gene expression) can be mentioned. Protective effects on cognitive functions, the immune system, lungs, heart, digestive organs, hematopoietic organs, testes, skin, and cartilage tissues have been reported when administering hydrogen-enriched water to small laboratory animals. In patients undergoing radiation therapy for therapeutic purposes, the intake of such enriched water reduced side effects without affecting the primary treatment outcome. Inhalation of gaseous H2 in terminal-stage cancer patients improved hematopoietic function [8].
Among substances of plant origin, celastrol (tripterin) has been described. This is a pentacyclic triterpenoid from the quinone methide family, derived from the roots of Chinese medicinal plants (Tripterygium wilfordii or Celastrus regelii). According to the authors, this substance is capable of inhibiting NF-κB pathways, exhibiting antioxidant activity, suppressing lipid peroxidation and oxidative DNA damage, and increasing animal survival in experiments [9].
As a separate research direction, the administration of bacterial strains to enhance survival has been described. In particular, data were published showing that the introduction of a radioresistant variant of St. aureus followed by irradiation of animals at the LD100/30 level increased survival by 77.7% [10]. It is assumed that the modified strains actively synthesize antioxidant factors affecting various organs and systems, which collectively enhance the body’s resistance.
Since the creation of an original low-molecular-weight chemical compound with multi-targeted actions and diverse biological activities presents a significant challenge, research groups have long adopted the strategy of utilizing natural creations. In this regard, cytokines, hormones, and vitamins have been studied as a means for the prevention and early therapy of radiation injuries [5]. Over a decade ago, the U.S. Food and Drug Administration (US FDA) approved a number of radioprotective agents (radiomitigators), including 5-androstenediol (neumune), genistein (BIO 300), protein kinase ON01210 (Ex-RAD), Toll-like receptor 5 agonist CBLB502 (entolimod), the corticosteroid beclomethasone (OrbeShield®), recombinant human interleukin-12 (HemaMax®), and recombinant growth factor G-CSF (Neupogen®) [5]. An analogue of the latter agent (filgrastim) is also positioned as a means for the pathogenetic therapy of acute radiation sickness [6], although it is essentially a genetically engineered granulocyte colony-stimulating factor that stimulates leukopoiesis. Beta-leukin, which is no longer produced but was recommended as an effective radiomitigator for early use after accidental irradiation [5][6], is a recombinant analogue of human pro-inflammatory interleukin-1β. While the aforementioned natural compounds and their recombinant analogues demonstrate certain levels of radioprotective activity — either directly or indirectly (e.g., by stimulating an inflammatory response that counteracts processes developing during radiation sickness) — and protect specific organs and systems from the progression of radiation sickness, they still fail to provide a comprehensive protection for the entire organism.
According to a number of radiology specialists, the primary direction in the development of approaches to pharmacological correction of the initial response to irradiation is the creation of complex formulations. Their components should effectively target various organs and systems, and accordingly, different links in the pathogenesis of early disorders [5]. A consequence of this approach has been a striking diversity of means for preventing and treating individual manifestations of radiation sickness, albeit with questionable acceptability of the results from their combined use.
This diversity is exemplified by Chinese authors [11], who illustrated all radioprotective means mentioned in scientific publications (Fig. 1). Among the listed agents, along with “classical” amifostine, are the non-steroidal anti-inflammatory benzydamine for treating oral mucositis, glutamine for mucosal restoration, pentoxifylline with statins to reduce inflammation and prevent fibrosis, meloxicam as an anti-proliferative agent, and superoxide dismutase for protection against free radical damage. The authors also mentioned Toll-like receptor agonists, low-molecular nitroxide compounds, and sphingosine-1-phosphate.
The natural radioprotective agents described so far include plant extracts (flavonoids, etc.), vitamins (A, C, E), trace elements (selenium), and components of bacterial lysates (flagellin). Cytokines (including a number of pro-inflammatory cytokines and growth factors) and some immunomodulators (β-glucan and others) are separately listed, with stem cell therapy and gene therapy also mentioned [11].
It becomes absolutely evident that employing such a complex therapy with substances of multidirectional biological action is hazardous to health, even in the event of a direct threat to human life, as the cumulative side effects may well surpass the consequences of radiation exposure. This justifies the necessity of searching for more versatile medicinal methods of protection.
Properties of alpha-2-macroglobulin and prospects for its use as a means for prevention and treatment of radiation injuries
The trend toward using natural molecules with a medium or high molecular weight, which allow for a more diverse impact on the organism compared to low-molecular-weight chemical compounds, is evident. However, it has not reached its logical conclusion as high-molecular-weight blood plasma components with radioprotective properties are now rarely discussed in the scientific literature.
Meanwhile, for over half a billion years, a family of proteins that exerts a complex effect on the body’s organs and systems and possesses, among other things, pronounced radioprotective properties has existed. In humans, the main representative of this family is alpha-2-macroglobulin (α2M); its concentration in blood serum is about 2–3 g/L. In humans, this family also includes pregnancy-associated alpha-2-glycoprotein and plasma protein-A (their blood levels increase during pregnancy and in estrogen-dependent tumors, but even then, they are significantly lower than the concentration of α2M). In rodents, this family additionally includes murinoglobulins. Some authors also classify the complement components C3 and C4 as part of this superfamily.
Possessing a significant molecular weight (720 kDa), α2M can perform regulatory and transport functions in the intercellular environment. This protein is known to be biologically active; however, low-molecular-weight compounds diffuse rather slowly in the absence of fluid flow. This glycoprotein has four subunits in its structure, each containing a masked thiol ester that specifically binds a wide spectrum of proteinases while partially preserving their activity. Furthermore, the subunits have a fairly extensive hydrophobic region, which is also a binding site.
A unique property of α2M, along with its ability to form covalent and non-covalent bonds with a wide variety of compounds, consists in its capacity to change the conformation and accessibility of binding sites on its surface. A number of functions are triggered only after interaction with proteinases (an example of the α2M structure based on cryo-electron microscopy data is shown in Fig. 2). Such features determine the variability of its properties, even regarding its clearance rate from circulation; thus, the α2M complex with some cytokines can circulate in the body for a long time, while after interaction with a proteinase, the half-life of the complex is no more than 1.5 min. In general, the structure and functions of α2M, including those mentioned above, have been described in sufficient detail in the scientific literature from the 1980s to the present day [12–17].
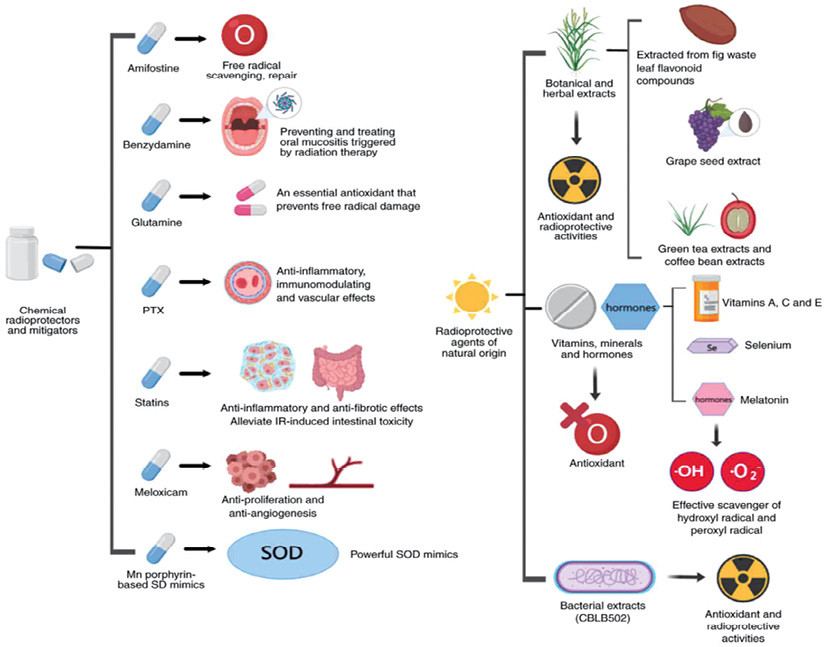

Figure prepared by the authors based on data from [11], CC BY license
Fig. 1. Main radioprotective agents: GM-CSF, granulocyte-macrophage colony stimulating factor; G-CSF, granulocyte colony stimulating factor; M-CSF, macrophage colony stimulating factor; IL, interleukin; TPO, thrombopoietin; M-GDF, megakaryocyte growth development factor; F L, Flt-3 ligand; TSLP, thymic stromal lymphopoietin; KGF, keratinocyte growth factor; HAPO, hemangiopoietin; LIF, leukemia inhibitor y factor; PF4, platelet factor 4; EPO, erythropoietin; SCF, stem cell factor; SDF-1, stromal cell-derived factor-1; MSC, mesenchymal stem cell; AED, 5-androstenone
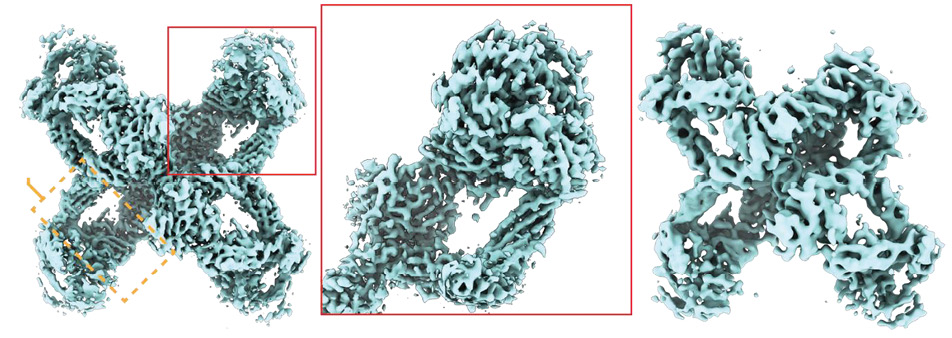
Figure prepared by the authors based on data from [17], CC BY license
Fig. 2. Structure of the native and transformed (after interaction with a proteinase) alpha-2-macroglobulin molecule according to cryo-electron microscopy data
Among the properties of this protein directly or indirectly involved in radioprotection mechanisms is the fact that α2M subunits are paired by two zinc atoms and can interact with various metals via a competitive mechanism [18]. This protein is capable of performing its functions through different types of receptors expressed by various cell types, including endocytosis receptors (the low-density lipoprotein receptor family, or so-called LRP receptors) and signaling receptors (including GRP-78, classified as a heat shock protein). It has been previously established that α2M is involved in lipid metabolism, tissue remodeling, regulation of enzymatic and antioxidant system functions, while controlling the development of an inflammatory response. The fact that α2M binds and transports many cytokines, and its synthesis is regulated by cytokines and growth factors (IL-6 stimulates synthesis, while TGF-β inhibits), as well as the circumstance that α2M influences the functions of leukocytes (primarily neutrophils), lymphocytes, and macrophages, and is actively involved in inflammatory, autoimmune, and proliferative processes [12–16], indicates that many effects of currently used radioprotectors are realized with the direct participation of this protein. Among other things, α2M can induce the activation of NF-κB signaling pathways [19] and is capable of interacting with histones [20]. Despite these promising observations, only a few publications have addressed the radioprotective properties of α2M.
The first attempts found in the scientific literature to use α2M as a radioprotective agent date back to the 1960s. In 1967, the research team [21] showed that alpha-macroglobulin fractions (19S), isolated by zonal ultracentrifugation from the blood serum of rats and mice and administered both separately and in a mixture, increased the survival of mice irradiated at a dose of 750 roentgen. It was shown that the «murine» α2M fraction stimulates hematopoiesis; thus, differences in hematopoietic activity in irradiated (400 roentgen) mice receiving the macroglobulin fraction compared to the control averaged 3–5 times in the bone marrow and 9–10 times in the spleen. At the same time, the administration of a fraction of isologous proteins with a lower molecular weight did not produce a similar effect, underscoring the importance of using precisely native, undamaged preparations of high-molecular-weight proteins.
In 1974, British researchers also reported on the ability of α2M to provide radioprotection, including as part of fractions or preparations containing IgA impurities. Mice were irradiated at a sublethal dose of 500 rad and administered α2M or its containing protein fraction containing 4 h after whole-body irradiation, with a second administration 4 days later. Human albumin was used as a reference protein. It was found that the administration of α2M contributed to an increase in the total number of leukocytes. Since an unphysiologically high dose (20 mg), on the contrary, had a suppressive effect, a dose of 5 mg was considered promising. It was suggested that earlier administration (less than 4 h after radiation exposure) would be more effective [22].
Attempts to use α2M as a radioprotective agent were also made in Russia. In particular, in 1995, a patent was registered for an invention of a method for obtaining a blood plasma fraction containing α2M and intended, among other things, for the treatment of radiation injuries [23]. Despite a rather primitive and controversial production method (the fraction is essentially a mixture of α2M and IgM), the effectiveness of the development was demonstrated in clinical studies involving cancer patients undergoing chemo- and radiotherapy. The obtained preparation was administered intramuscularly multiple times. In patients additionally receiving the preparation alongside their treatment, the frequency of leukopenia and thrombocytopenia decreased, general well-being improved, the number of inflammatory infiltrates decreased, and in some cases, a regression of metastases was observed [23].
Serbian scientists published a series of works on the radioprotective properties of α2M in 2003, 2009, and 2011. The initial experiments were conducted on rats irradiated at a dose of 6.7 Gy. Amifostine was used as a reference drug. The α2M purification method included chromatography on DEAE-cellulose and gel filtration. It was demonstrated that the prophylactic administration of α2M provided 100% protection against lethal outcome at the specified radiation dose, as well as amifostine. In that experiment, a mixture of amifostine and α2M best preserved the total number of leukocytes and platelets [24].
In another study, the same research group administered α2M in physiological saline at a dose of 4.5 mg per rat weighing 200–250 g, 30 min after irradiation at 6.7 Gy. In the untreated group, about 50% of the animals died within the 4-week observation period, while in the groups receiving amifostine or α2M, all animals survived and showed weight gain. In irradiated animals without treatment, the relative liver weight (calculated as the ratio of the organ’s absolute weight to the animal’s body weight) decreased. Conversely, the administration of α2M and amifostine increased the relative weight, peaking in differences at 14 days. When studying morphological changes in the liver tissues of irradiated animals, the administration of α2M and amifostine minimized damage and prevented the formation of necrotic foci [25].
It was experimentally established that both α2M and amifostine significantly reduced the number of DNA damages in irradiated animals (although not fully normalizing this indicator). A comparable influence in the direction and magnitude of amifostine and α2M action on superoxide dismutase activity, the expression of the universal transcription factor NF-κB, and changes in serum IL-6 concentration in rats upon irradiation was observed. On this basis, the authors concluded that the radioprotective efficacy of α2M results from a combination of several mechanisms of action, each with its own effectiveness. It is possible that a number of the protective effects of amifostine are due to its ability to stimulate the synthesis of α2M. Thus, α2M is a central effector of natural radioprotection, at least in rats [26, 27].
These findings are of particular interest given the toxicity of amifostine, while a number of its radioprotective effects are mediated by the activity of a non-toxic protein (α2M), whose synthesis it stimulates.
In 2018, Liu et al. conducted experiments on cell cultures to demonstrate that α2M has a beneficial effect on the differentiation and proliferation of irradiated bone tissue cells, reduced autophagy, lowered oxidative stress levels, and decreased apoptosis activity, exhibiting pronounced radioprotective effects [28]. In 2022, other Chinese researchers, Huangfu et al., published data confirming the restoration of functions and maintenance of viability in irradiated fibroblasts, as well as a reduction in oxidative stress levels under the influence of α2M. Mitochondrial damage caused by irradiation was reduced with α2M, presumably by inhibiting the loss of mitochondrial membrane potential, calcium expression, and TRPM2 [29].
The significance of α2M and its receptor LRP1 (CD91) in the progression of malignant neoplasms has been repeatedly confirmed. It is suggested that restoring α2M homeostasis in tumors to levels characteristic of healthy tissues may suppress the tumor’s ability to evade immune surveillance and promote cancer cell death [30]. Since α2M levels and the activity of LRP receptor expression are directly interrelated with the growth activity of a number of malignant tumors, organismal aging, and a general decrease in resistance to external influences, it is evident that a number of teratogenic effects observed in radiation injuries may also be regulated by influencing the content of this protein in the body.
It is noteworthy that available literature contains almost no publications from Western European and North American scientists dedicated to studying the radioprotective properties of α2M. We found only one study by US researchers, who demonstrated that individuals with high levels of α2M in their blood tolerate therapeutically prescribed irradiation more easily [31].
Among the literature reviews summarizing information on the radioprotective properties of α2M, an article by Chinese specialists described its possible mechanisms of action, including the ability to stimulate antioxidant enzyme activity, prevent the development of fibrosis, maintain homeostasis and hemodynamic equilibrium, and improve DNA repair and cell recovery processes [32].
It is important to note that one of the possible reasons for the scarcity of scientific studies on the radioprotective properties of α2M relates to the difficulty in isolating highly purified preparations of α2M from blood with preserved structure and activity. Since aggressive solvents (e.g., acetonitrile) and elution buffers with acidic pH destroy the protein’s structure, chromatography methods using HPLC are hardly applicable, as well as the attempts to obtain recombinant proteins enabling the purification of α2M via affinity chromatography [33]. The most acceptable methods are gentle, multi-step preparative low-pressure chromatography techniques.
Danish specialists made a significant contribution to the study and development of methods for the preparative isolation of α2M. Between 1970 and 1990, results obtained via an extensive series of studies dedicated to this protein and other members of its family were published, including descriptions of its structure, mechanisms of interaction with receptors and ligands [34–36]. The primary method proposed for isolating α2M from blood involved the removal of plasminogen by polyethylene glycol precipitation, zinc-chelate chromatography, followed by gel filtration and concentration via ultrafiltration [37]. A study of α2M and its receptors, which requires obtaining native α2M preparations in acceptable quantities, has also been undertaken by scientists from the USA [20], Germany [13], Argentina [19], and Russia [38–40]. A patent has been registered for an invention describing two stages of zinc-chelate chromatography used to isolate this protein from blood [41]. A methodological approach for obtaining α2M preparations has been published, involving the removal of plasminogen on lysine-sepharose, polyethylene glycol precipitation, anion-exchange chromatography, zinc-chelate chromatography, and gel filtration [42]. Some authors have attempted to simplify the production methods and integrate them with general blood processing approaches, as well as to increase their safety, which is already a significant step forward [43, 44]. However, the described method of obtaining α2M from the so-called Cohn fraction IV cannot be considered perfect from the perspective of the quality of the resulting protein.
In any case, methodological approaches to obtaining preparations of native α2M require adaptation for the needs of industrial production of blood-derived drugs. However, no publications have appeared thus far on the deep processing of blood serum and plasma that would enable the production of such blood preparations, beyond the “standard” list recommended for practical use in clinical practice: albumin, protein fraction (the same albumin with impurities), a number of proteins affecting blood coagulation (fibrinogen, thrombin, antihemophilic globulin, fibrinolysin, cryoprecipitate components), and certain classes of immunoglobulins.1
CONCLUSION
Alpha-2-macroglobulin (α2M) is a promising radioprotective agent and a key component of innate radioresistance. The administration of this protein into the organism reduces lethality and oxidative stress levels, protects DNA from damage, mitigates the severity of leukopenia and thrombocytopenia, and decreases the number of necrotic foci. Further research into the radioprotective properties of this protein and the optimization of its isolation methods from blood for industrial-scale production are required.
1 Beloborodov VA, Kelchevskaya EA. Transfusion of blood and its components: A Study Guide. Irkutsk State Medical University of the Ministry of Health of the Russian Federation. Irkutsk: ISMU; 2020 (In Russ.).
References
1. Supotnitskiy MV. Nuclear war as it looks. Journal of NBC Protection Corps. 2023;7(3):205–36 (In Russ.). https://doi.org/10.35825/2587-5728-2023-7-3-205-235
2. Indjic DR. Remediation of the areas contaminated by depleted uranium ammunition. Military Technical Courier. 2021;69(1):230–52. EDN: HFRRZX
3. Cheng C, Chen L, Guo K, Xie J, Shu Y, He S, et al. Progress of uranium-contaminated soil bioremediation technology. Journal of Environmental Radioactivity. 2022;241:106773. https://doi.org/10.1016/j.jenvrad.2021.106773
4. Gladkikh VD, Balandin NV, Basharin VA, Belovolov AYu, Grebenyuk AN, Druzhkov AV, et al. Status and prospects of development of means of prevention and treatment of radiation injuries. Ed. of Gladkikh VD. Moscow: Commentary; 2017 (In Russ.).
5. Grebenyuk AN, Gladkikh VD. Modern Condition and prospects of medicines for prevention and early treatment of radiation injures. Radiation Biology. Radioecology. 2019;59(2):132–49 (In Russ.). https://doi.org/10.1134/S0869803119020085
6. Vasin MV. B-190 (indralin) in the light of history of formation of ideas of the mechanism of action of radioprotectors. Radiation Biology. Radioecology 2020;60(4):378–95 (In Russ.). https://doi.org/10.31857/S0869803120040128
7. Zhang J, Li K, Zhang Q, Zhu Z, Huang G, Tian H. Polycysteine as a new type of radio-protector ameliorated tissue injury through inhibiting ferroptosis in mice. Cell Death and Disease. 2021;12(2):195. https://doi.org/10.1038/s41419-021-03479-0
8. Hirano S, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F. Molecular hydrogen as a potential clinically applicable radioprotective agent. International Journal of Molecular Sciences. 2021;22(9):4566. https://doi.org/10.3390/ijms22094566
9. Wang H, Ahn KS, Alharbi SA, Shair OH, Arfuso F, Sethi G, et al. Celastrol alleviates gamma irradiation-induced damage by modulating diverse inflammatory mediators. International Journal of Molecular Sciences. 2020;21(3):1084. https://doi.org/10.3390/ijms21031084
10. Gaynutdinov TR, Ryzhkin SA, Shavaliev RF, Vagin KN, Kurbangaleev YM, Kalimullin FH, et al. Evaluation of anti-radiation efficacy of the Staphylococcus aureus-derived therapeutic agent. Extreme Medicine. 2024;6(2):63–75 (In Russ.). https://doi.org/10.47183/mes.2024.023
11. Liu L, Liang Z, Ma S, Li L, Liu X. Radioprotective countermeasures for radiation injury (Review). Molecular Medicine Reports. 2023;27(3):66.
12. Petersen CM. Alpha 2-macroglobulin and pregnancy zone protein. Serum level, alpha 2-macroglobuline receptors, cellular synthesis and aspects of function relation to immunology. Danish Medical Bulletin. 1993;40:409–46.
13. Birkenmeier G. Targetting the proteinase inhibitor and immune modulatory function of human alpha 2-macroglobulin. Mod. Asp. Immunobiol. 2001;2:32–6.
14. Zorin NA, Zorina VN. Macroglobulin signaling system. Biomedical Chemistry. 2012;58(4):400–10 (In Russ.).
15. Zorina VN, Zorin NA. Evolution and mechanisms for implementing functions of the regulatory system of proteins belonging to the macroglobulin family. Advances in Current Biology. 2013;133(3):284–91 (In Russ.). EDN: QYZWJN
16. Vandooren J, Itoh Y. Alpha-2-Macroglobulin in inflammation, immunity and infections. Frontiers in Immunology. 2021;12:803244. https://doi.org/10.3389/fimmu.2021.803244
17. Arimura Y, Funabiki H. Structural mechanics of the Alpha-2Macroglobulin transformation. Journal of Molecular Biology. 2022;434(5):167413. https://doi.org/10.1016/j.jmb.2021.167413
18. Zorina VN, Evdokimova EA, Rejniuk VL. Assessing the possibility of interactions of various metals with alpha-2-macroglobulin and other human blood proteins in vitro. Extreme Medicine. 2023;25(2):105–11 (In Russ.). https://doi.org/10.47183/mes.2023.011
19. Cáceres LC, Bonacci GR, Sánchez MC, Chiabrando GA. Activated α(2) macroglobulin induces matrix metalloproteinase 9 expression by low-density lipoprotein receptorrelated protein 1 through MAPK-ERK1/2 and NF-κB activation in macrophage-derived cell lines. Journal of Cellular Biochemistry. 2010;111(3):607–17. https://doi.org/10.1002/jcb.22737
20. Chu CT, Howard GC, Misra UK, Pizzo SV. Alpha 2-macroglobulin: a sensor for proteolysis. Annals of the New York Academy of Sciences. 1994;737:291–307. https://doi.org/10.1111/j.1749-6632.1994.tb44319.x
21. Hanna MG, Nettesheim P, Fisher WD, Peters LC, Francis MW. Serum alpha globulin fraction: survival-and-recovery effect in irradiated mice. Science. 1967;157(3795):1458–61.
22. Tunstall AM, James K. The effect of human alpha 2-macroglobulin on the restoration of humoral responsiveness in x-irradiated mice. Clinical and Experimental Immunology. 1975;21(1):173–80.
23. Tereshchenko IP, Murashova NS, Shalnova GA. Method for obtaining a substance for the treatment of tumors, radiation injuries and toxic infectious conditions. Patent of the Russian Federation No. 2042953; 1995 (In Russ.). EDN: CJUSLD
24. Sevaljević L, Dobrić S, Bogojević D, Petrović M, Koricanać G, Vulović M, Kanazir D, Ribarac-Stepić N. The radioprotective activities of turpentine-induced inflammation and alpha2macroglobulin: the effect of dexamethasone on the radioprotective efficacy of the inflammation. Journal of Radiation Research. 2003;44(1):59–67. https://doi.org/10.1269/jrr.44.59
25. Mihailović M, Milosević V, Grigorov I, Poznanović G, IvanovićMatić S, Grdović N, et al. The radioprotective effect of alpha2macroglobulin: a morphological study of rat liver. Medical Science Monitor. 2009;15(7):BR188–93.
26. Mihailović M, Dobrić S, Poznanović G, Petrović M, Uskoković A, Arambasić J, et al. The acute-phase protein alpha2-macroglobulin plays an important role in radioprotection in the rat. Shock. 2009;31(6):607–14. https://doi.org/10.1097/shk.0b013e31818bb625
27. Bogojević D, Poznanović G, Grdović N, Grigorov I, Vidaković M, Dinić S, et al. Administration of rat acute-phase protein alpha(2)-macroglobulin before total-body irradiation initiates cytoprotective mechanisms in the liver. Radiation and Environmental Biophysics. 2011;50(1):167–79. https://doi.org/10.1007/s00411-010-0331-z
28. Liu Y, Cao W, Kong X, Li J, Chen X, Ge Y, et al. Protective effects of alpha-2-macroglobulinon human bone marrow mesenchymal stem cells in radiation injury. Molecular Medicine Reports. 2018;18(5):4219–28. https://doi.org/10.3892/mmr.2018.9449
29. Huangfu C, Tang N, Yang X, Gong Z, Li J, Jia J, et al. Improvement of irradiation-induced fibroblast damage by alpha2-macroglobulin through alleviating mitochondrial dysfunction. Pharmaceutical Biology. 2022;60(1):1365–73. https://doi.org/10.1080/13880209.2022.2096077
30. Olbromski M, Mrozowska M, Piotrowska A, Kmiecik A, Smolarz B, Romanowicz H, et al. Prognostic significance of alpha-2-macrglobulin and low-density lipoprotein receptorrelated protein-1 in various cancers. American Journal of Cancer Research. 2024;14(6):3036–58. https://doi.org/10.62347/VUJV9180
31. von Reibnitz D, Yorke ED, Oh JH, Apte AP, Yang J, Pham H, et al. Predictive modeling of thoracic radiotherapy toxicity and the potential role of serum alpha-2-macroglobulin. Frontiers in Oncology. 2020;10:1395. https://doi.org/10.3389/fonc.2020.01395
32. Chen X, Kong X, Zhang Z, Chen W, Chen J, Li H, et al. Alpha2-macroglobulin as a radioprotective agent: a review. Clinical Journal of Cancer Research. 2014;26(5):611–21. https://doi.org/10.3978/j.issn.1000-9604.2014.09.04
33. Gupalova TV, Bormotova EA. Use of a recombinant protein that binds albumin and immunoglobulin G for proteomics purposes. Patent of the Russian Federation No. 2758604; 2021 (In Russ.). EDN: OINWVX
34. Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. The Journal of Biological Chemistry. 1989;264(20):11539–42.
35. Sottrup-Jensen L, Stepanik TM, Kristensen T, Wierzbicki DM, Jones CM, Lønblad PB, et al. Primary structure of human alpha 2-macroglobulin. V. The complete structure. The Journal of Biological Chemistry. 1984;259(13):8318–27.
36. Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Letters. 1990;276(1– 2):151–5. https://doi.org/10.1016/0014-5793(90)80530-v
37. Sottrup-Jensen L, Petersen TE, Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Letters. 1980;121(2):275–9. https://doi.org/10.1016/0014-5793(80)80361-9
38. Zorin NA, Zhabin SG, Chirikova TS. Changes in a2macroglobulins during evolution. Journal of Evolutionary Biochemistry and Physiology. 1990;26(3):289 (In Russ.).
39. Zorin NA, Zhabin SG, Belogorlova TI, Arkhipova SV. Possible similarity between α2-macroglobulin and pregnancy-dependent α2-glycoprotein and protein A: comparative study. Problems of Medical Chemistry. 1991;37:48–50 (In Russ.).
40. Doropheikov VV, Freidlin TS, Shcherbak IG. Human alpha2-macroglobulin as a main cytokine-binding plasma protein. Medical Immunology. 1999;1(5):5–12 (In Russ.).
41. Zorin NA, Zhabin SG. Method for isolation of alpha-macroglobulin and alpha-glycoprotein associated with pregnancy from blood plasma. Patent of the Russian Federation No. RU 2000809; 1993 (In Russ.). EDN: CTLDGH
42. Zorin NA, Zorina RM, Zorina VN. Production of alpha-2-macroglobulin preparations with desired properties. Russian Journal of Hematology and Transfusiology. 2000;5:20–1 (In Russ.).
43. Huangfu C, Ma Y, Lv M, Jia J, Zhao X, Zhang J. Purification of alpha2-macroglobulin from Cohn Fraction IV by immobilized metal affinity chromatography: A promising method for the better utilization of plasma. Journal of Chromatography B. 2016;1025:68–75. https://doi.org/10.1016/j.jchromb.2016.05.013
44. Huangfu C, Zhao X, Lv M, Jia J, Zhu F, Wang R, et al. Inactivation of viruses during a new manufacturing process of alpha2-macroglobulin from Cohn Fraction IV by dry-heat treatment. Transfusion. 2016;56(9):2274–7. https://doi.org/10.1111/trf.13714
About the Authors
V. N. ZorinaRussian Federation
Veronika N. Zorina
St. Petersburg
E. A. Evdokimova
Russian Federation
Elena A. Evdokimova
St. Petersburg
Supplementary files
Review
For citations:
Zorina V.N., Evdokimova E.A. Prospects for the use of alpha-2-macroglobulin as a radioprotective agent. Extreme Medicine. 2025;27(4):516-524. https://doi.org/10.47183/mes.2025-316

















